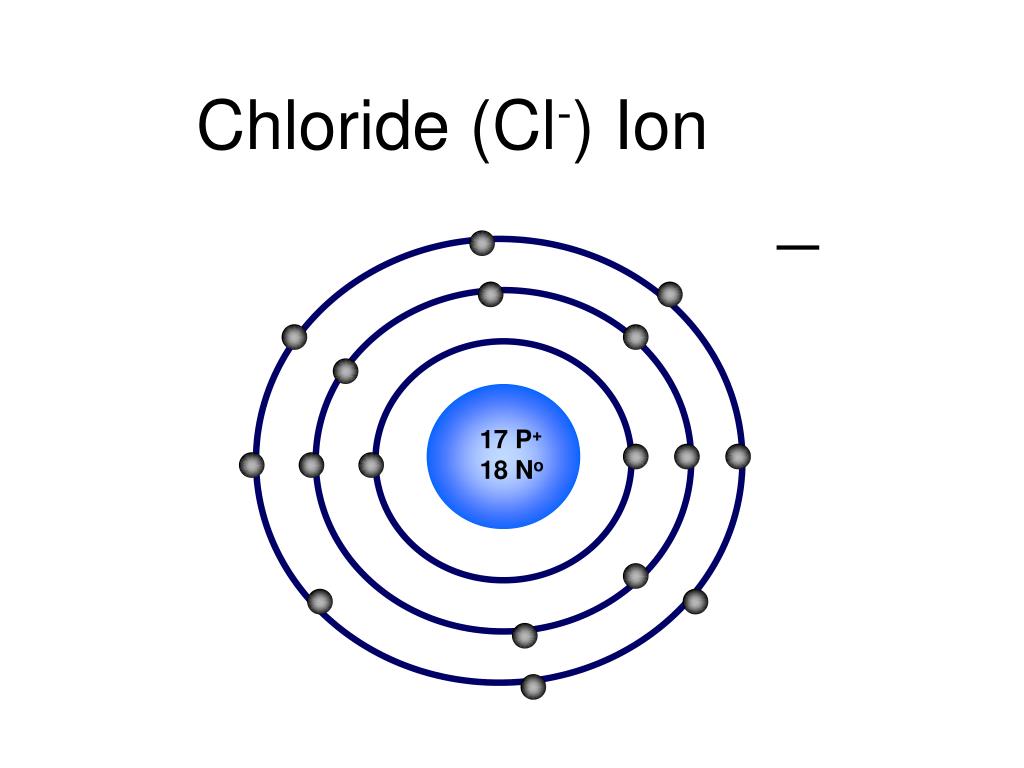
Cl Ion Formula . Because most metals form cations and. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. Most cations have charges of [+1] through [+6]. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The formula for an ionic compound follows several conventions. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. The name of a metal ion is the. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. First, the cation is written before the anion. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an.
from www.slideserve.com
The formula for an ionic compound follows several conventions. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. The name of a metal ion is the. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. First, the cation is written before the anion. Because most metals form cations and. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Most cations have charges of [+1] through [+6]. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion.
PPT Seawater Chemistry PowerPoint Presentation, free download ID1073512
Cl Ion Formula Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. First, the cation is written before the anion. The formula for an ionic compound follows several conventions. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Most cations have charges of [+1] through [+6]. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Because most metals form cations and. The name of a metal ion is the.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Cl Ion Formula Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. The name of a metal ion is the. Most cations have charges of [+1]. Cl Ion Formula.
From chemistry-dictionary.yallascience.com
Formula unit of NaCl, sodium chloride Chemistry Dictionary Cl Ion Formula The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. Most cations have charges of [+1] through [+6]. First, the cation is written before the anion. The formula for an ionic. Cl Ion Formula.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine atom chemical reaction Cl Ion Formula The formula for an ionic compound follows several conventions. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Because most metals form cations and. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. The name of a metal ion is the. It. Cl Ion Formula.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image & Art Alamy Cl Ion Formula The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. The name of a metal ion is the. The formula for an ionic compound. Cl Ion Formula.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free download ID2090187 Cl Ion Formula The name of a metal ion is the. The formula for an ionic compound follows several conventions. Because most metals form cations and. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts,. Cl Ion Formula.
From exoxfkxkh.blob.core.windows.net
Chloride Ion In Water at Ronald Bourne blog Cl Ion Formula In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number. Cl Ion Formula.
From mungfali.com
Sodium Chloride Molecule Diagram Cl Ion Formula It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The formula for an ionic compound follows several conventions. First, the cation is written before the anion. The name. Cl Ion Formula.
From www.youtube.com
Draw the Lewis Structure of LiCl (Lithium Chloride) YouTube Cl Ion Formula The formula for an ionic compound follows several conventions. Because most metals form cations and. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. Most cations have charges of [+1] through [+6]. It has the same number of electrons as atoms of the preceding noble gas, argon,. Cl Ion Formula.
From byjus.com
Ammonium Chloride Formula Chemical and Structural Formula Cl Ion Formula In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Because most metals form cations and. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. First, the cation is written before the anion. The formula for an ionic. Cl Ion Formula.
From www.slideserve.com
PPT Seawater Chemistry PowerPoint Presentation, free download ID1073512 Cl Ion Formula First, the cation is written before the anion. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. The formula for an ionic compound follows several conventions. Most cations have charges of [+1] through [+6]. Because most metals form cations and. The formula of an ionic compound represents. Cl Ion Formula.
From molekula.com
Purchase Sodium chloride [7647145] online • Catalog • Molekula Group Cl Ion Formula First, the cation is written before the anion. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. Most cations have charges of [+1] through [+6]. Because most metals form cations and. In the solid state, ionic compounds are in crystal lattice containing many ions each of the. Cl Ion Formula.
From www.alamy.com
Sodium chloride is an ionic compound with the chemical formula NaCl, representing a 1 to 1 ratio Cl Ion Formula The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. The formula for an ionic compound follows several conventions. Because most metals form cations and. Chloride ion is a chlorine anion. Cl Ion Formula.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Cl Ion Formula Because most metals form cations and. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is. Cl Ion Formula.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in Cl Ion Formula Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. It has the same number of electrons as atoms of the preceding noble gas, argon, and. Cl Ion Formula.
From exygryntw.blob.core.windows.net
Chlorine Formula For Ion at Angela Reilly blog Cl Ion Formula Most cations have charges of [+1] through [+6]. The name of a metal ion is the. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. The formula for. Cl Ion Formula.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free download ID4520433 Cl Ion Formula Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. It has the same number of electrons as atoms of the preceding noble gas,. Cl Ion Formula.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl (Chloride ion) YouTube Cl Ion Formula Because most metals form cations and. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. The name of a metal ion is the.. Cl Ion Formula.
From www.youtube.com
Calculating Ion Concentrations in Solution YouTube Cl Ion Formula The formula for an ionic compound follows several conventions. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. The name of a metal ion is the. Most cations have charges of [+1] through [+6]. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is. Cl Ion Formula.
From studylib.net
Ionic Compounds Naming Cl Ion Formula Most cations have charges of [+1] through [+6]. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. Because most metals form cations and. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. In the solid state, ionic compounds are. Cl Ion Formula.
From www.youtube.com
Draw the Lewis Structure of CaCl2 (calcium chloride) YouTube Cl Ion Formula The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. The formula for an ionic compound follows several conventions. Because most metals form cations and. Instead,. Cl Ion Formula.
From saylordotorg.github.io
Ions Cl Ion Formula The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The name. Cl Ion Formula.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Cl Ion Formula It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. The name of a metal ion is the. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Thus, \(\ce{nacl}\) is the chemical formula for sodium. Cl Ion Formula.
From www.youtube.com
ClO Lewis Structure How to Draw the Lewis Structure for ClO YouTube Cl Ion Formula Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. Because most metals form cations and. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts,. Cl Ion Formula.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Molecules Combine with Cl Ion Formula The name of a metal ion is the. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. It has the same number of. Cl Ion Formula.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Cl Ion Formula Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. The formula for an ionic compound follows several conventions. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Chloride ion is a chlorine anion that forms the negatively charged part. Cl Ion Formula.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Cl Ion Formula Most cations have charges of [+1] through [+6]. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. The name of a metal ion is the. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple. Cl Ion Formula.
From chemistry291.blogspot.com
Chemical Formula for Sodium ChlorideSodium Chloride Formula Cl Ion Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. In the solid state, ionic compounds are in crystal lattice containing many ions each. Cl Ion Formula.
From chemistry291.blogspot.com
Chemical Formula for Sodium ChlorideSodium Chloride Formula Cl Ion Formula Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. In the solid state, ionic compounds are in crystal lattice containing many ions each of the. Cl Ion Formula.
From schematicmanualhertz.z19.web.core.windows.net
Cl Lewis Structure Diagram Cl Ion Formula Most cations have charges of [+1] through [+6]. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. The formula for an ionic compound follows several conventions. The formula of an ionic compound represents. Cl Ion Formula.
From pasuukantujuh.blogspot.com
Cl Dot Structure Ionic Bonding CK12 Foundation / To draw the lewis dot structure for Cl Ion Formula Because most metals form cations and. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and. Cl Ion Formula.
From www.youtube.com
CaCl2 Lewis Structure How to draw the Lewis Dot Structure for Calcium Chloride YouTube Cl Ion Formula Most cations have charges of [+1] through [+6]. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Because most metals form cations and.. Cl Ion Formula.
From drmchemistrytutor.com
sodium chloride ions Dr. M. Chemistry Tutor Cl Ion Formula Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. The formula for an ionic compound follows several conventions. First, the cation is written. Cl Ion Formula.
From www.slideserve.com
PPT Ions, Ionic Bonds, and Metallic Bonds PowerPoint Presentation, free download ID2973921 Cl Ion Formula Thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. The formula for an ionic compound follows several conventions. Chloride ion is a chlorine anion that. Cl Ion Formula.
From socratic.org
What is the chemical equation for HCl dissolving into water and ionizing? Socratic Cl Ion Formula The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized ca2+ ca 2 +. Most cations have charges of [+1] through [+6]. First, the cation is written before the anion.. Cl Ion Formula.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Cl Ion Formula The name of a metal ion is the. Chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Most cations have charges of [+1] through [+6]. It. Cl Ion Formula.