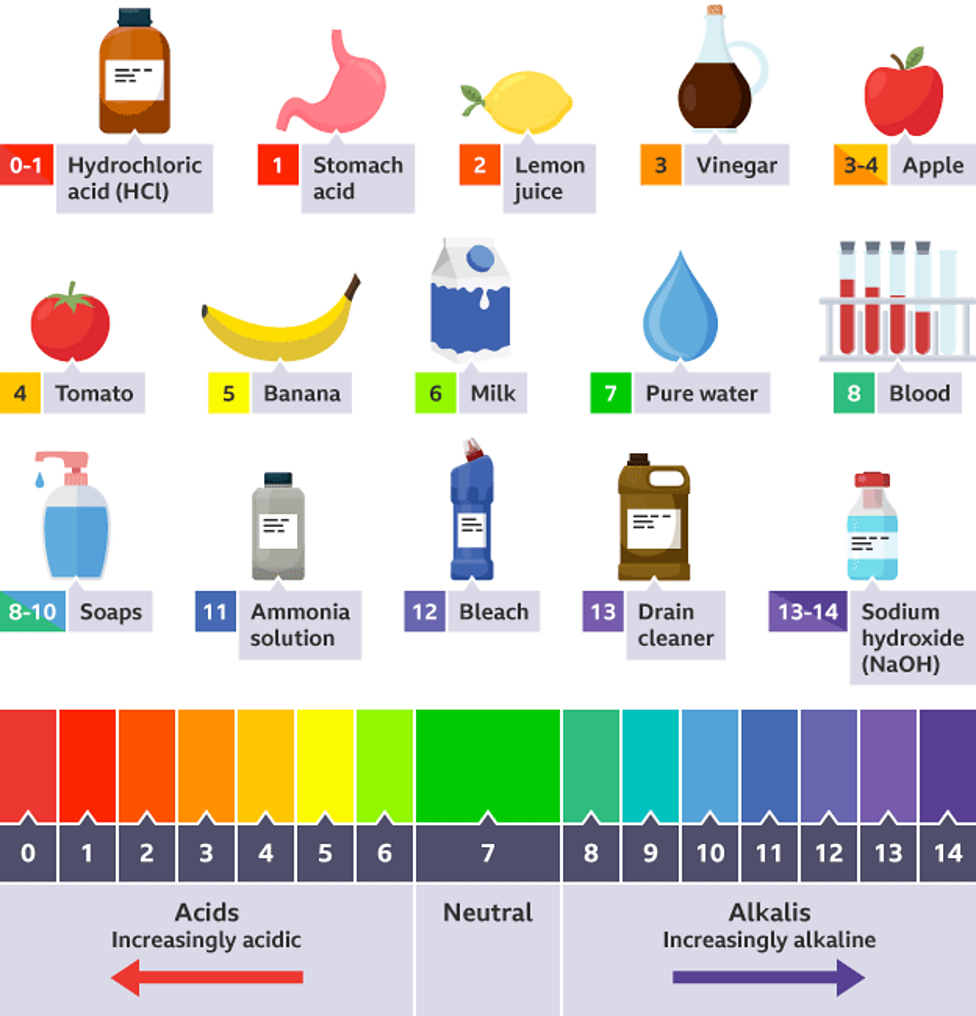
Why Is The Ph Of Distilled Water Usually 7 . When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. Pure distilled water starts with a neutral ph of 7. But why is the ph of distilled water not neutral ph 7? In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. This does not mean the. The ph of distilled water is theoretically about 7. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. What ph is safe for drinking. Why does the ph of a substance matter? Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. A ph value below 7 indicates acidity, with lower values indicating stronger acidity.
from www.bbc.co.uk
In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. A ph value below 7 indicates acidity, with lower values indicating stronger acidity. But why is the ph of distilled water not neutral ph 7? If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. Why does the ph of a substance matter? What ph is safe for drinking. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. Pure distilled water starts with a neutral ph of 7.
What is the pH scale and what does it measure? BBC Bitesize
Why Is The Ph Of Distilled Water Usually 7 But why is the ph of distilled water not neutral ph 7? This does not mean the. Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. But why is the ph of distilled water not neutral ph 7? The ph of distilled water is theoretically about 7. If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. What ph is safe for drinking. Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. Why does the ph of a substance matter? Pure distilled water starts with a neutral ph of 7. A ph value below 7 indicates acidity, with lower values indicating stronger acidity.
From bahamaswater.in
How do you determine what pH level is safe for drinking? Why Is The Ph Of Distilled Water Usually 7 Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. A ph value below 7 indicates acidity, with lower values indicating stronger acidity. The ph of distilled water is theoretically about 7. Purified water from distillation, deionisation or reverse osmomsis purification systems. Why Is The Ph Of Distilled Water Usually 7.
From www.researchgate.net
pH of distilled water and pH 7 buffer solution at various temperatures Why Is The Ph Of Distilled Water Usually 7 Pure distilled water starts with a neutral ph of 7. If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. The ph of distilled water is theoretically about 7. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. Conversely,. Why Is The Ph Of Distilled Water Usually 7.
From www.bbc.co.uk
What is the pH scale and what does it measure? BBC Bitesize Why Is The Ph Of Distilled Water Usually 7 Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. Why does the ph of a substance matter? In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. Pure water has a ph of 7 and is considered “neutral” because. Why Is The Ph Of Distilled Water Usually 7.
From www.intec-america.com
Things You Must Know About pH Control and Drinking Water Treatment Why Is The Ph Of Distilled Water Usually 7 Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. But why is the ph of distilled water not neutral ph 7? A ph value below 7 indicates acidity, with lower values indicating stronger acidity. If you want to know “what is the ph of distilled water?”, the answer depends on. Why Is The Ph Of Distilled Water Usually 7.
From www.tardish2o.co.uk
PH water Levels pH level in Drinking Water What is a safe pH value? Why Is The Ph Of Distilled Water Usually 7 In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. A ph value below 7 indicates acidity, with lower values indicating stronger acidity. Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. Purified water from distillation,. Why Is The Ph Of Distilled Water Usually 7.
From www.slideserve.com
PPT Description of experiment PowerPoint Presentation, free download Why Is The Ph Of Distilled Water Usually 7 Why does the ph of a substance matter? This does not mean the. If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. Purified water from distillation, deionisation or reverse. Why Is The Ph Of Distilled Water Usually 7.
From watertechadvice.com
Alkaline Water vs. Distilled Water (Which is Best?) Why Is The Ph Of Distilled Water Usually 7 The ph of distilled water is theoretically about 7. In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. Conversely, ph values above 7 indicate alkalinity, with higher values indicating. Why Is The Ph Of Distilled Water Usually 7.
From www.waterev.com
What is the PH of Distilled Water What is the PH value of water and Why Is The Ph Of Distilled Water Usually 7 If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. Why does the ph of a substance matter? Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. The ph of distilled water is theoretically about 7.. Why Is The Ph Of Distilled Water Usually 7.
From www.waterev.com
What is the PH of Distilled Water? Is Distilled Water Acidic Or Basic Why Is The Ph Of Distilled Water Usually 7 The ph of distilled water is theoretically about 7. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. Conversely, ph values above 7 indicate alkalinity, with higher values indicating. Why Is The Ph Of Distilled Water Usually 7.
From perfect-hydration.com
Drinking Water pH Explained What Should Be the Ideal pH? Perfect Why Is The Ph Of Distilled Water Usually 7 If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. The ph of distilled water is theoretically about 7. Conversely, ph values above 7 indicate alkalinity, with higher values indicating. Why Is The Ph Of Distilled Water Usually 7.
From netsolwater.com
Why is pH important in measuring or determining water quality Why Is The Ph Of Distilled Water Usually 7 But why is the ph of distilled water not neutral ph 7? If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. This does not mean the. The ph of distilled water is theoretically about 7. A ph value below 7 indicates acidity, with lower values. Why Is The Ph Of Distilled Water Usually 7.
From www.eseasongear.com
pH in Distilled Water Why Is The Ph Of Distilled Water Usually 7 What ph is safe for drinking. Why does the ph of a substance matter? Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. When exposed to air , it absorbs carbon dioxide, lowering. Why Is The Ph Of Distilled Water Usually 7.
From www.geologycafe.com
Geology Why Is The Ph Of Distilled Water Usually 7 A ph value below 7 indicates acidity, with lower values indicating stronger acidity. What ph is safe for drinking. Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. Pure water has a ph. Why Is The Ph Of Distilled Water Usually 7.
From mypurewater.com
What is the pH of Distilled Water? My Pure Water Why Is The Ph Of Distilled Water Usually 7 This does not mean the. In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. But why is the ph of distilled water not neutral ph 7? What ph is. Why Is The Ph Of Distilled Water Usually 7.
From www.awesomewaterfilters.com.au
pH of Distilled Water Understanding Its Importance Awesome Water Filters Why Is The Ph Of Distilled Water Usually 7 Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. Pure distilled water starts with a neutral ph of 7. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. The ph of distilled water is theoretically about 7. Pure water has a ph of 7 and is considered “neutral” because it. Why Is The Ph Of Distilled Water Usually 7.
From aquaclearws.com
Why Is The pH of Water Important? Aqua Clear Water Systems Why Is The Ph Of Distilled Water Usually 7 But why is the ph of distilled water not neutral ph 7? Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. This does not mean the. Conversely, ph values above 7 indicate alkalinity, with. Why Is The Ph Of Distilled Water Usually 7.
From www.vevor.com
What is the pH of Distilled Water and Why Does It Matter? VEVOR Blog Why Is The Ph Of Distilled Water Usually 7 Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. A ph value below 7 indicates acidity, with lower values indicating stronger acidity. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. Pure distilled water starts with a neutral ph of 7. But why. Why Is The Ph Of Distilled Water Usually 7.
From aqualife.ca
Discover the power of pH on your health Aqualife Why Is The Ph Of Distilled Water Usually 7 Why does the ph of a substance matter? The ph of distilled water is theoretically about 7. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. But why is the ph of distilled water not neutral ph 7? In practice, it’s difficult to put an exact number on the ph of distilled water. Why Is The Ph Of Distilled Water Usually 7.
From brainly.in
what is the pH of distilled water and common salt solution Brainly.in Why Is The Ph Of Distilled Water Usually 7 Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. Why does the ph of a substance matter? If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. But why is the ph of distilled water not neutral ph 7? When exposed to air. Why Is The Ph Of Distilled Water Usually 7.
From mywaterearth.com
Safe PH Level For Drinking Water MyWaterEarth&Sky Why Is The Ph Of Distilled Water Usually 7 In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. Pure distilled water starts with a neutral ph of 7. Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. Conversely, ph values above 7 indicate alkalinity,. Why Is The Ph Of Distilled Water Usually 7.
From sciencetrends.com
What Is The pH Of Distilled Water? Science Trends Why Is The Ph Of Distilled Water Usually 7 When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. Why does the ph of a substance matter? Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. What ph is safe for drinking. If you want to know “what is the ph of distilled water?”, the answer. Why Is The Ph Of Distilled Water Usually 7.
From www.slideserve.com
PPT Description of experiment PowerPoint Presentation, free download Why Is The Ph Of Distilled Water Usually 7 Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. But why is the ph of distilled water not neutral ph 7? The ph of distilled water is theoretically about 7. Pure water has. Why Is The Ph Of Distilled Water Usually 7.
From www.vevor.com
What is the pH of Distilled Water and Why Does It Matter? VEVOR Blog Why Is The Ph Of Distilled Water Usually 7 But why is the ph of distilled water not neutral ph 7? In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. This does not mean the. What ph is safe for drinking. Why. Why Is The Ph Of Distilled Water Usually 7.
From svalbardi.com
What to Know about the pH of Drinking Water Svalbarði Polar Iceberg Water Why Is The Ph Of Distilled Water Usually 7 When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. A ph value below 7 indicates acidity, with lower values indicating stronger acidity. Why does the ph of a substance matter? What ph is safe for drinking. The ph of distilled water is theoretically about 7. Purified water from distillation, deionisation or reverse osmomsis. Why Is The Ph Of Distilled Water Usually 7.
From www.medicalnewstoday.com
What to know about the pH of water Why Is The Ph Of Distilled Water Usually 7 What ph is safe for drinking. But why is the ph of distilled water not neutral ph 7? Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. Why does the ph of a substance matter? Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. If you want. Why Is The Ph Of Distilled Water Usually 7.
From skfelixer.com
The pH Value Of Purified Water — All You Need To Know SKF Elixer Why Is The Ph Of Distilled Water Usually 7 What ph is safe for drinking. The ph of distilled water is theoretically about 7. This does not mean the. Why does the ph of a substance matter? Pure distilled water starts with a neutral ph of 7. But why is the ph of distilled water not neutral ph 7? If you want to know “what is the ph of. Why Is The Ph Of Distilled Water Usually 7.
From www.youtube.com
What is th pH of distilled water? YouTube Why Is The Ph Of Distilled Water Usually 7 What ph is safe for drinking. Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. But why is the ph of distilled water not neutral ph 7? If you want to know “what is the ph of distilled water?”, the answer. Why Is The Ph Of Distilled Water Usually 7.
From www.slideserve.com
PPT Description of experiment PowerPoint Presentation, free download Why Is The Ph Of Distilled Water Usually 7 If you want to know “what is the ph of distilled water?”, the answer depends on the amount of time it’s exposed to air. The ph of distilled water is theoretically about 7. Conversely, ph values above 7 indicate alkalinity, with higher values indicating greater. Pure distilled water starts with a neutral ph of 7. Why does the ph of. Why Is The Ph Of Distilled Water Usually 7.
From www.awesomewaterfilters.com.au
What Is The pH Of Distilled Water and Tap Water? Why Is The Ph Of Distilled Water Usually 7 The ph of distilled water is theoretically about 7. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to.. Why Is The Ph Of Distilled Water Usually 7.
From www.waterev.com
What is the pH Of Distilled Water? Why Is The Ph Of Distilled Water Usually 7 A ph value below 7 indicates acidity, with lower values indicating stronger acidity. Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. Pure distilled water starts with a neutral ph of 7. What ph is safe for drinking. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually. Why Is The Ph Of Distilled Water Usually 7.
From drowwater.com
Best pH Level for Drinking Water? DrowWater Why Is The Ph Of Distilled Water Usually 7 When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide. Conversely, ph values above. Why Is The Ph Of Distilled Water Usually 7.
From www.vevor.com
What is the pH of Distilled Water and Why Does It Matter? VEVOR Blog Why Is The Ph Of Distilled Water Usually 7 Purified water from distillation, deionisation or reverse osmomsis purification systems will usually have an acidic ph. Why does the ph of a substance matter? But why is the ph of distilled water not neutral ph 7? In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide.. Why Is The Ph Of Distilled Water Usually 7.
From mungfali.com
PH Scale Chart Water Why Is The Ph Of Distilled Water Usually 7 A ph value below 7 indicates acidity, with lower values indicating stronger acidity. Pure water has a ph of 7 and is considered “neutral” because it has neither acidic nor basic qualities. Why does the ph of a substance matter? This does not mean the. In practice, it’s difficult to put an exact number on the ph of distilled water. Why Is The Ph Of Distilled Water Usually 7.
From mungfali.com
Ph Scale Of Water Why Is The Ph Of Distilled Water Usually 7 But why is the ph of distilled water not neutral ph 7? When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. Why does the ph of a substance matter? In practice, it’s difficult to put an exact number on the ph of distilled water because simple exposure to air can dissolve carbon dioxide.. Why Is The Ph Of Distilled Water Usually 7.
From educationcrises.z4.web.core.windows.net
Ph Of Distilled Water Why Is The Ph Of Distilled Water Usually 7 This does not mean the. Why does the ph of a substance matter? Pure distilled water starts with a neutral ph of 7. But why is the ph of distilled water not neutral ph 7? When exposed to air , it absorbs carbon dioxide, lowering the ph to around 5.5 to. What ph is safe for drinking. Purified water from. Why Is The Ph Of Distilled Water Usually 7.