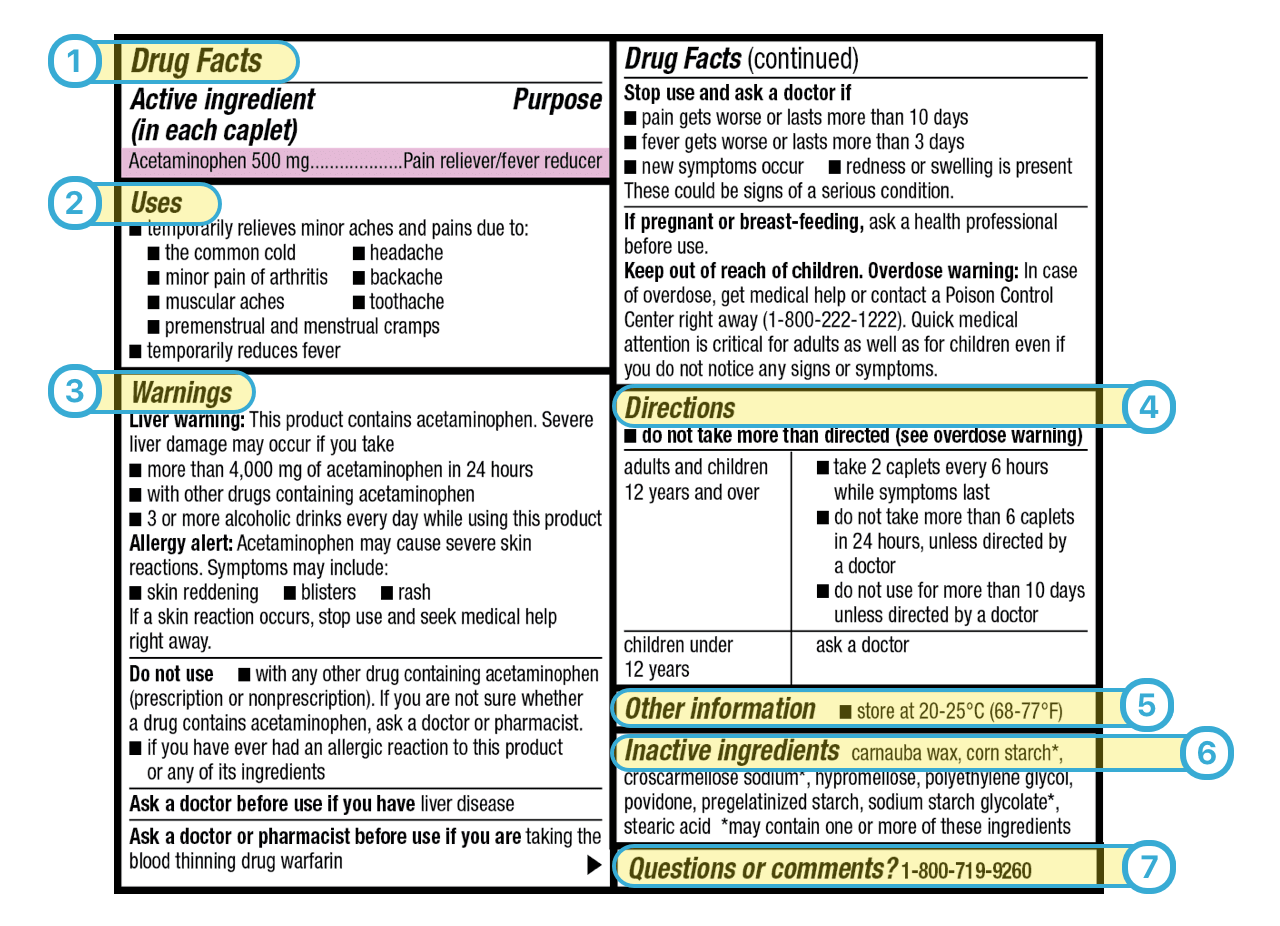
Drug Labeling Fda Guidance . Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. An anda is required to contain information to show. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Search by name, download all labels, or access.
from www.drugwatch.com
This guidance is intended to assist applicants of human prescription drug and biological products in determining the. An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. Find the most recent drug and device labeling information submitted to fda by companies. Search by name, download all labels, or access.
How to Read OvertheCounter and Prescription Drug Labels
Drug Labeling Fda Guidance The goal of this initiative was to reduce. An anda is required to contain information to show. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. Search by name, download all labels, or access.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Drug Labeling Fda Guidance An anda is required to contain information to show. Find the most recent drug and device labeling information submitted to fda by companies. Search by name, download all labels, or access. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. This guidance is intended. Drug Labeling Fda Guidance.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Drug Labeling Fda Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Search by name, download all labels, or access. Find the most recent drug and device labeling information submitted to fda by companies. The goal of this. Drug Labeling Fda Guidance.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Labeling Fda Guidance The goal of this initiative was to reduce. Search by name, download all labels, or access. An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended. Drug Labeling Fda Guidance.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Labeling Fda Guidance The goal of this initiative was to reduce. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Search by name, download. Drug Labeling Fda Guidance.
From www.europeanpharmaceuticalreview.com
FDA releases guidance on pharmaceutical product labelling Drug Labeling Fda Guidance An anda is required to contain information to show. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find the most recent drug and device labeling information submitted to fda by companies. Search by name,. Drug Labeling Fda Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labeling Fda Guidance Search by name, download all labels, or access. Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. An anda is required to contain information to show. The goal of this initiative was to reduce. In june 2006, the. Drug Labeling Fda Guidance.
From www.studypool.com
SOLUTION Fda guidelines on labelling of drug product reviewer Studypool Drug Labeling Fda Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Search by name, download all labels, or access. An anda is required to contain information to show. The goal of this initiative was to reduce. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find. Drug Labeling Fda Guidance.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Labeling Fda Guidance In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. An anda is required to contain information to show. The goal of. Drug Labeling Fda Guidance.
From pharmdevgroup.com
Lear About Drug product labeling FDA consulting PDG Drug Labeling Fda Guidance An anda is required to contain information to show. Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. Search by name, download all labels, or access. This guidance is intended. Drug Labeling Fda Guidance.
From www.fda.gov
Sample Drug Facts Label FDA Drug Labeling Fda Guidance The goal of this initiative was to reduce. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Search by name, download all labels, or access. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. An anda is required to contain information to show. Find. Drug Labeling Fda Guidance.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Drug Labeling Fda Guidance Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. An anda is required to contain information to show. Search by name, download all labels, or access. In june 2006, the fda made changes to the pharmaceutical labeling requirements. Drug Labeling Fda Guidance.
From www.fda.gov
The OvertheCounter Medicine Label Take a Look FDA Drug Labeling Fda Guidance The goal of this initiative was to reduce. Search by name, download all labels, or access. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. An anda is required to contain information to show. Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended. Drug Labeling Fda Guidance.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Drug Labeling Fda Guidance An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. The goal of this initiative was to reduce. Search by name, download all labels, or access. Find. Drug Labeling Fda Guidance.
From medshadow.org
How to Read a Drug Label, According to a Pharmacist MedShadow Drug Labeling Fda Guidance The goal of this initiative was to reduce. Search by name, download all labels, or access. An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended. Drug Labeling Fda Guidance.
From www.celegence.com
FDA Guidance on Navigating Annual Reportable Labeling Changes Drug Labeling Fda Guidance The goal of this initiative was to reduce. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find the most recent drug and device labeling information submitted to fda by companies. An anda is required to contain information to show. Search by name, download all labels, or access. This guidance is intended. Drug Labeling Fda Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labeling Fda Guidance Search by name, download all labels, or access. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find the most recent drug and device labeling information submitted to fda by companies. An anda is required to contain information to show. The goal of this initiative was to reduce. This guidance is intended. Drug Labeling Fda Guidance.
From www.fda.gov
How Do I Use Prescription Drug Labeling FDA Drug Labeling Fda Guidance Find the most recent drug and device labeling information submitted to fda by companies. The goal of this initiative was to reduce. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Search by name, download all labels, or access. An anda is required to contain information to show. In june 2006, the. Drug Labeling Fda Guidance.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Drug Labeling Fda Guidance Search by name, download all labels, or access. The goal of this initiative was to reduce. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Find the most recent drug and device labeling information submitted to fda by companies. An anda is required to contain information to show. In june 2006, the. Drug Labeling Fda Guidance.
From www.fda.gov
OTC Drug Facts Label FDA Drug Labeling Fda Guidance The goal of this initiative was to reduce. An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended to assist applicants of human prescription drug and. Drug Labeling Fda Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labeling Fda Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Find the most recent drug and device labeling information submitted to fda by companies. An anda is required to contain information to show. Search by name, download all labels, or access. In june 2006, the fda made changes to the pharmaceutical labeling requirements. Drug Labeling Fda Guidance.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Drug Labeling Fda Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. An anda is required to contain information to show. Search by name, download all labels, or access. Find the most recent drug and device labeling information. Drug Labeling Fda Guidance.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Labeling Fda Guidance The goal of this initiative was to reduce. Search by name, download all labels, or access. An anda is required to contain information to show. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find. Drug Labeling Fda Guidance.
From www.finnegan.com
Final FDA Guidance on Safety Considerations for Medication Container Drug Labeling Fda Guidance The goal of this initiative was to reduce. Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Search by name, download all labels, or access. An anda is required to contain information to show. This guidance is intended. Drug Labeling Fda Guidance.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Drug Labeling Fda Guidance Find the most recent drug and device labeling information submitted to fda by companies. An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. This guidance is intended to assist applicants of human prescription drug and. Drug Labeling Fda Guidance.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Drug Labeling Fda Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Find the most recent drug and device labeling information submitted to fda by companies. An anda is required to contain information to show. The goal of this initiative was to reduce. Search by name, download all labels, or access. In june 2006, the. Drug Labeling Fda Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labeling Fda Guidance Search by name, download all labels, or access. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. An anda is required to contain information to show. Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended. Drug Labeling Fda Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labeling Fda Guidance An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of this initiative was to reduce. Find the most recent drug and device labeling information submitted to fda by companies. Search by name, download all labels, or access. This guidance is intended. Drug Labeling Fda Guidance.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Labeling Fda Guidance Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. An anda is required to contain information to show. The goal of. Drug Labeling Fda Guidance.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Drug Labeling Fda Guidance Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. An anda is required to contain information to show. The goal of this initiative was to reduce. Search by name, download all labels, or access. This guidance is intended. Drug Labeling Fda Guidance.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Drug Labeling Fda Guidance In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. An anda is required to contain information to show. The goal of this initiative was to reduce. Search by name, download all labels, or access. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Find. Drug Labeling Fda Guidance.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Drug Labeling Fda Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. An anda is required to contain information to show. Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. The goal of. Drug Labeling Fda Guidance.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Drug Labeling Fda Guidance Search by name, download all labels, or access. The goal of this initiative was to reduce. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. Find. Drug Labeling Fda Guidance.
From www.slideshare.net
Fda proposes new guide for drug labeling Drug Labeling Fda Guidance An anda is required to contain information to show. Find the most recent drug and device labeling information submitted to fda by companies. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Search by name,. Drug Labeling Fda Guidance.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Drug Labeling Fda Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. In june 2006, the fda made changes to the pharmaceutical labeling requirements for drugs and biological products. An anda is required to contain information to show. Search by name, download all labels, or access. The goal of this initiative was to reduce. Find. Drug Labeling Fda Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labeling Fda Guidance Find the most recent drug and device labeling information submitted to fda by companies. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Search by name, download all labels, or access. An anda is required to contain information to show. In june 2006, the fda made changes to the pharmaceutical labeling requirements. Drug Labeling Fda Guidance.