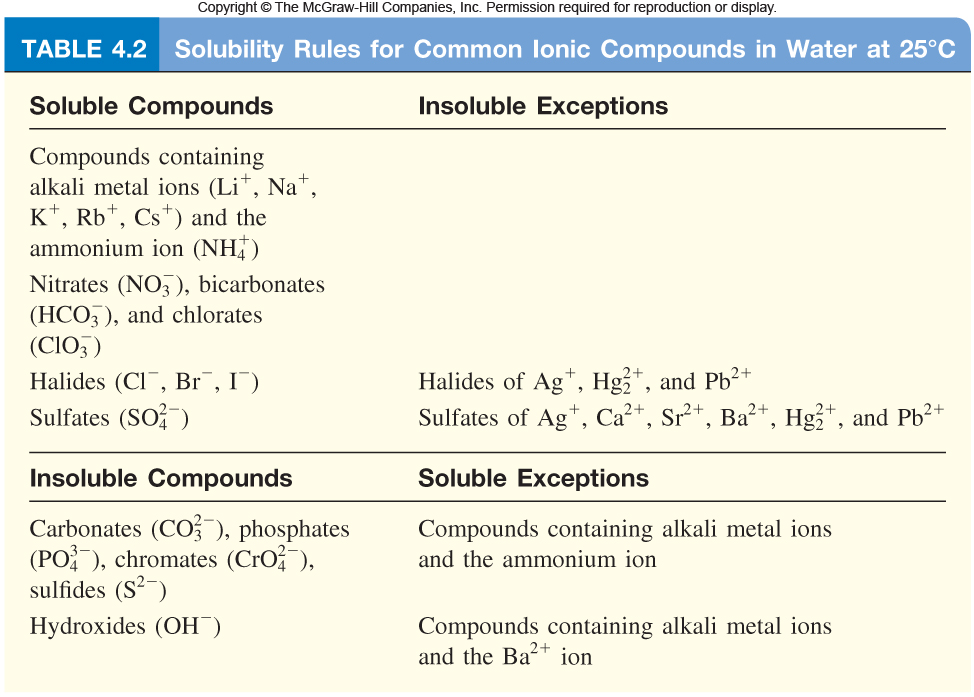
Is Lead Hydroxide A Salt . Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. The most common method involves reacting lead. Acid + metal hydroxide → salt + water. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Soluble salts can be made by reacting acids with soluble or insoluble reactants. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Titration must be used if the reactants are soluble.
from socratic.org
This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Acid + metal hydroxide → salt + water. The most common method involves reacting lead. Titration must be used if the reactants are soluble. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Soluble salts can be made by reacting acids with soluble or insoluble reactants. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made.
Which of the following substances are insoluble in water? Socratic
Is Lead Hydroxide A Salt Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Acid + metal hydroxide → salt + water. The most common method involves reacting lead. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Is Lead Hydroxide A Salt Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Pb2 + (aq) + 2nh 3(aq) +. Is Lead Hydroxide A Salt.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Is Lead Hydroxide A Salt Soluble salts can be made by reacting acids with soluble or insoluble reactants. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Acid + metal hydroxide → salt + water. The most common method involves reacting lead. If a small amount of sodium. Is Lead Hydroxide A Salt.
From www.youtube.com
Equation for PbSO4 + H2O Lead (II) sulfate + Water YouTube Is Lead Hydroxide A Salt If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Titration must be used if the reactants are soluble. The most common method involves reacting lead. Acid + metal hydroxide → salt + water. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than. Is Lead Hydroxide A Salt.
From mariabsmitho.blob.core.windows.net
Lead Hydroxide Reaction Equation at mariabsmitho blog Is Lead Hydroxide A Salt Acid + metal hydroxide → salt + water. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Titration must be used if the reactants are soluble. Lead (ii) hydroxide is generally produced through the. Is Lead Hydroxide A Salt.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Is Lead Hydroxide A Salt This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Acid + metal hydroxide → salt + water. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) hydroxide is generally produced through. Is Lead Hydroxide A Salt.
From trendzhit.com
Neutralisation Reaction Defination, Types, Equation, Applications Is Lead Hydroxide A Salt Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Lead (ii) hydroxide is generally produced through the reaction. Is Lead Hydroxide A Salt.
From chem.libretexts.org
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts Is Lead Hydroxide A Salt If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Lead (ii) ion reacts with aqueous ammonia to precipitate a white. Is Lead Hydroxide A Salt.
From 88guru.com
What are Salts in Chemistry Characteristics, Types 88guru Is Lead Hydroxide A Salt This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. The most common method involves reacting lead. Acid + metal hydroxide → salt + water. Lead (ii) hydroxide is generally produced through the reaction of lead. Is Lead Hydroxide A Salt.
From fyojqwyda.blob.core.windows.net
Lead Hydroxide Reaction at Jonathan Ertl blog Is Lead Hydroxide A Salt Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids. Is Lead Hydroxide A Salt.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Is Lead Hydroxide A Salt Soluble salts can be made by reacting acids with soluble or insoluble reactants. The most common method involves reacting lead. Acid + metal hydroxide → salt + water. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: This page looks at the formation of some. Is Lead Hydroxide A Salt.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium Is Lead Hydroxide A Salt If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. The. Is Lead Hydroxide A Salt.
From slideplayer.com
Lecture 0502 Types of Reactions. 1. Synthesis elements Is Lead Hydroxide A Salt Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: The most common method involves reacting lead. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) hydroxide. Is Lead Hydroxide A Salt.
From www.indiamart.com
Lab Grade Lead(II) Hydroxide Acetate Anhydrous Emplura (MERCK), 98 Is Lead Hydroxide A Salt Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: The most common method involves reacting lead. Acid + metal hydroxide → salt + water. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Titration must be used if. Is Lead Hydroxide A Salt.
From slideplayer.com
Lecture 0502 Types of Reactions. 1. Synthesis elements Is Lead Hydroxide A Salt Acid + metal hydroxide → salt + water. The most common method involves reacting lead. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an. Is Lead Hydroxide A Salt.
From slideplayer.com
Chapter 1 Intro to Chemistry ppt download Is Lead Hydroxide A Salt Titration must be used if the reactants are soluble. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Acid + metal hydroxide →. Is Lead Hydroxide A Salt.
From quizlet.com
Solubility Rules Diagram Quizlet Is Lead Hydroxide A Salt Soluble salts can be made by reacting acids with soluble or insoluble reactants. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt,. Is Lead Hydroxide A Salt.
From www.researchgate.net
Precipitation of Hydroxide Salts Download Scientific Diagram Is Lead Hydroxide A Salt Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Acid + metal hydroxide → salt + water.. Is Lead Hydroxide A Salt.
From www.slideserve.com
PPT Unit 2 Elements and Compounds Atoms, Molecules & Ions Is Lead Hydroxide A Salt Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. The most common method involves reacting lead. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. When acids react with metal. Is Lead Hydroxide A Salt.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Is Lead Hydroxide A Salt Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Soluble salts can be made by reacting acids with soluble or insoluble reactants. The most common method involves reacting lead. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using. Is Lead Hydroxide A Salt.
From www.slideshare.net
SPM Form 4 Chapter 8 Salt Is Lead Hydroxide A Salt Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected. Is Lead Hydroxide A Salt.
From www.slideserve.com
PPT Acid Definitions PowerPoint Presentation ID6398687 Is Lead Hydroxide A Salt Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Titration must be used if the reactants are soluble. Lead (ii) hydroxide is generally produced through the reaction of lead. Is Lead Hydroxide A Salt.
From slideplayer.com
Nomenclature And Classifying Compounds Lesson ppt download Is Lead Hydroxide A Salt Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Titration must be used if the reactants are soluble. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide:. Is Lead Hydroxide A Salt.
From www.slideshare.net
Acids And Bases Is Lead Hydroxide A Salt Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Titration must be. Is Lead Hydroxide A Salt.
From sciencelab.co.ke
Lead Hydroxide Sciencelab limited Is Lead Hydroxide A Salt Titration must be used if the reactants are soluble. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii). Is Lead Hydroxide A Salt.
From www.alamy.com
Lead hydroxide precipitate formed by adding sodium hydroxide (NaOH) to Is Lead Hydroxide A Salt Acid + metal hydroxide → salt + water. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Soluble salts can be made by reacting acids with soluble or insoluble reactants. The most common method involves reacting lead. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Lead (ii) hydroxide is generally. Is Lead Hydroxide A Salt.
From www.indiamart.com
Lead Hydroxide, 1319466 at Rs 1074 in Mumbai ID 24632765533 Is Lead Hydroxide A Salt Acid + metal hydroxide → salt + water. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Pb2 + (aq) + 2nh 3(aq). Is Lead Hydroxide A Salt.
From socratic.org
Which of the following substances are insoluble in water? Socratic Is Lead Hydroxide A Salt When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Acid + metal hydroxide → salt + water. Soluble salts can be made by reacting acids with soluble or insoluble. Is Lead Hydroxide A Salt.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation, free Is Lead Hydroxide A Salt When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Acid + metal hydroxide → salt + water. This page looks at the formation of some insoluble. Is Lead Hydroxide A Salt.
From www.tessshebaylo.com
Electrolysis Of Salt Water Half Equations Tessshebaylo Is Lead Hydroxide A Salt Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a. Is Lead Hydroxide A Salt.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Is Lead Hydroxide A Salt If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Titration must be used if the reactants are soluble. Lead (ii) hydroxide is generally produced through the reaction of lead (ii) salts with an alkali. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather. Is Lead Hydroxide A Salt.
From www.sciencephoto.com
Lead (II) Hydroxide Precipitate, 1 of 3 Stock Image C030/8102 Is Lead Hydroxide A Salt The most common method involves reacting lead. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Titration must be used if the reactants are soluble. When acids react with metal hydroxides (commonly. Is Lead Hydroxide A Salt.
From www.dreamstime.com
A Table of Lead Compounds Colors Stock Vector Illustration Is Lead Hydroxide A Salt If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Acid + metal hydroxide → salt + water. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: When acids react with metal hydroxides (commonly known as alkalis), a. Is Lead Hydroxide A Salt.
From www.indiamart.com
Lead (II) Hydroxide Acetate Anhydrous at Rs 880/gram Sugar Of Lead in Is Lead Hydroxide A Salt This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Titration must. Is Lead Hydroxide A Salt.
From www.youtube.com
Sodium Hydroxide + Sulfuric Acid Acid Base Neutralization Reaction Is Lead Hydroxide A Salt When acids react with metal hydroxides (commonly known as alkalis), a salt and water are made. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Titration must be used if the reactants are soluble. If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white.. Is Lead Hydroxide A Salt.
From www.sciencephoto.com
Lead (II) hydroxide precipitate, 3 of 3 Stock Image C036/3123 Is Lead Hydroxide A Salt If a small amount of sodium hydroxide solution is added to colorless lead(ii) nitrate solution, a white. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Lead (ii) hydroxide is generally produced through the reaction. Is Lead Hydroxide A Salt.