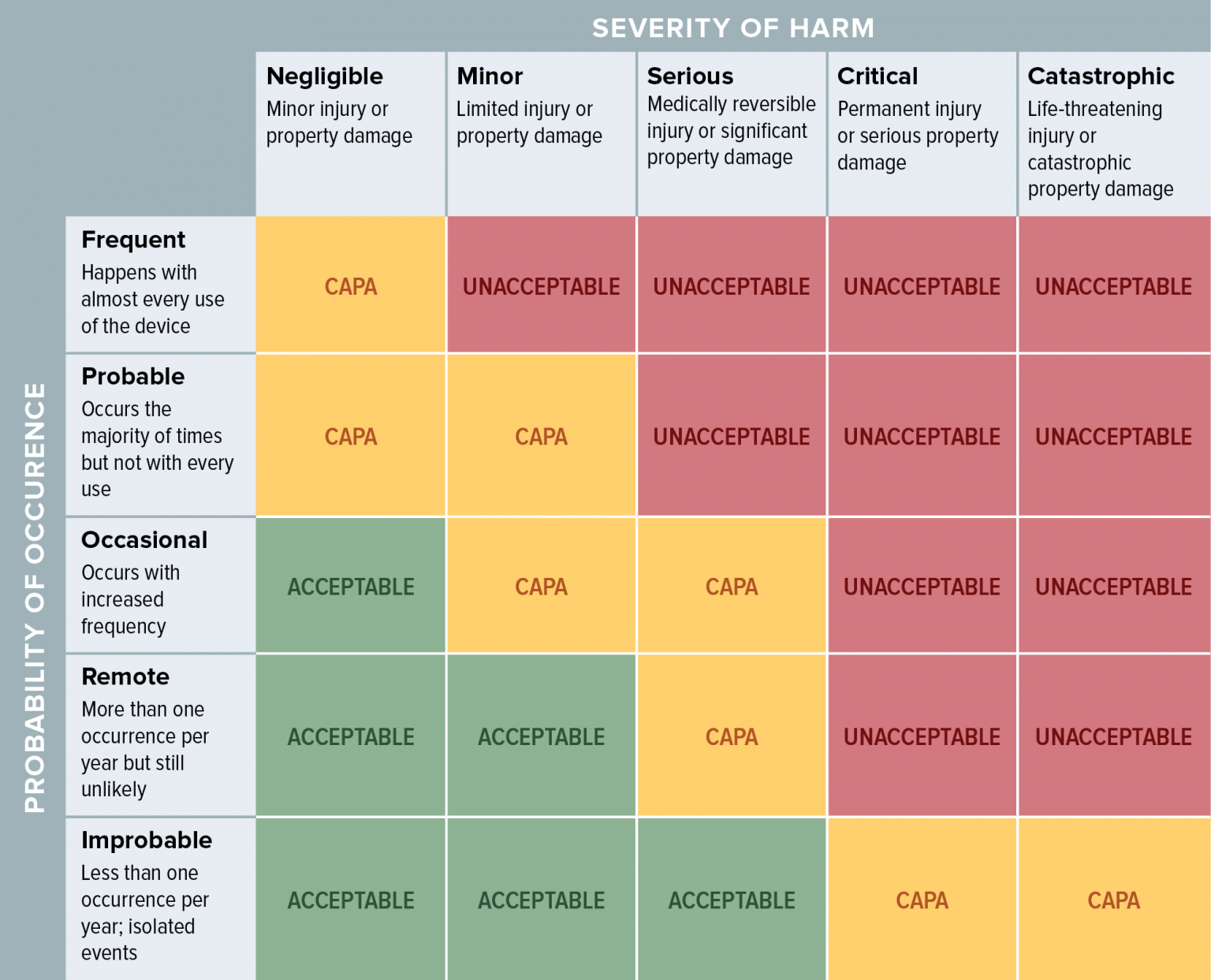
Medical Device Control Strategy . The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient.
from www.orielstat.com
Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26.
Creating a Medical Device Risk Management Plan and Doing Analysis
Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient.
From www.meddeviceonline.com
Navigating The Universe Of Risk In Medical Device Development Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.slideteam.net
Medical Device Development And Commercialization Process PPT PowerPoint Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From studylib.net
Medical Devices Management Policy V7.0 Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From www.researchgate.net
1Medical device risk management strategy (Adapted from ISO 14971 Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From medicaldevicehq.com
How to write instructions for use for medical devices Medical Device HQ Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.scribd.com
Clcgp004 Medical Devices Governance Management Policy v6 Medical Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.cognidox.com
Medical Device Development Guide Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.greenlight.guru
Definitive Guide to Change Management for Medical Devices Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.desertcart.in
Buy Medical Device Quality Management Systems Strategy and Techniques Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.dotcompliance.com
Top 10 Medical Device Testing Companies of 2021 Dot Compliance Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.pinterest.co.kr
The ITL Medical Device Product Development Process Integrated Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.youtube.com
Automating Medical Device Management YouTube Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.otherarticles.com
Winning The Medical Device Market How To Build A Successful Gtm Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From starfishmedical.com
5 Top Annual Plan Medical Device Design and Development Process Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From tsqasia.com
Medical Devices EU Design Control Process Support TSQ Middle East & Asia Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From www.slideteam.net
Effective Medical Device Sales Strategy PPT Presentation Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.nbautomation.com
Medical devices' management and traceability NB Automation Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.qualio.com
Ultimate guide to medical device design controls Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From templates.rjuuc.edu.np
Regulatory Strategy Template For Medical Devices Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.researchgate.net
Medical device lifecycle management (see online version for colours Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From cashier.mijndomein.nl
Medical Device Regulatory Strategy Template Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.
From www.ebme.co.uk
Medical devices getting management policies right Medical Device Control Strategy Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Medical Device Control Strategy.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Control Strategy The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. Medical Device Control Strategy.
From medicaldevicehq.com
Medical device design control terminology Medical Device HQ 1 Medical Device Control Strategy Medicinal products used in combination with a medical device (art 117) • mdr entered into application on 26. Focus is on successful practices and control strategies throughout the product lifecycle to ensure risk is commensurate with patient. The uk’s inaugural medical technology (medtech) strategy sets out how we will ensure the health and social care system. Medical Device Control Strategy.