Calorimeter Isolated System . Calorimetry is used to measure amounts of heat transferred to or from a substance. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. To do so, the heat is exchanged with a calibrated object. Calorimeters are carefully insulated so that heat transfer in or out is negligible. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there.
from schoolbag.info
A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. Calorimeters are carefully insulated so that heat transfer in or out is negligible. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter.
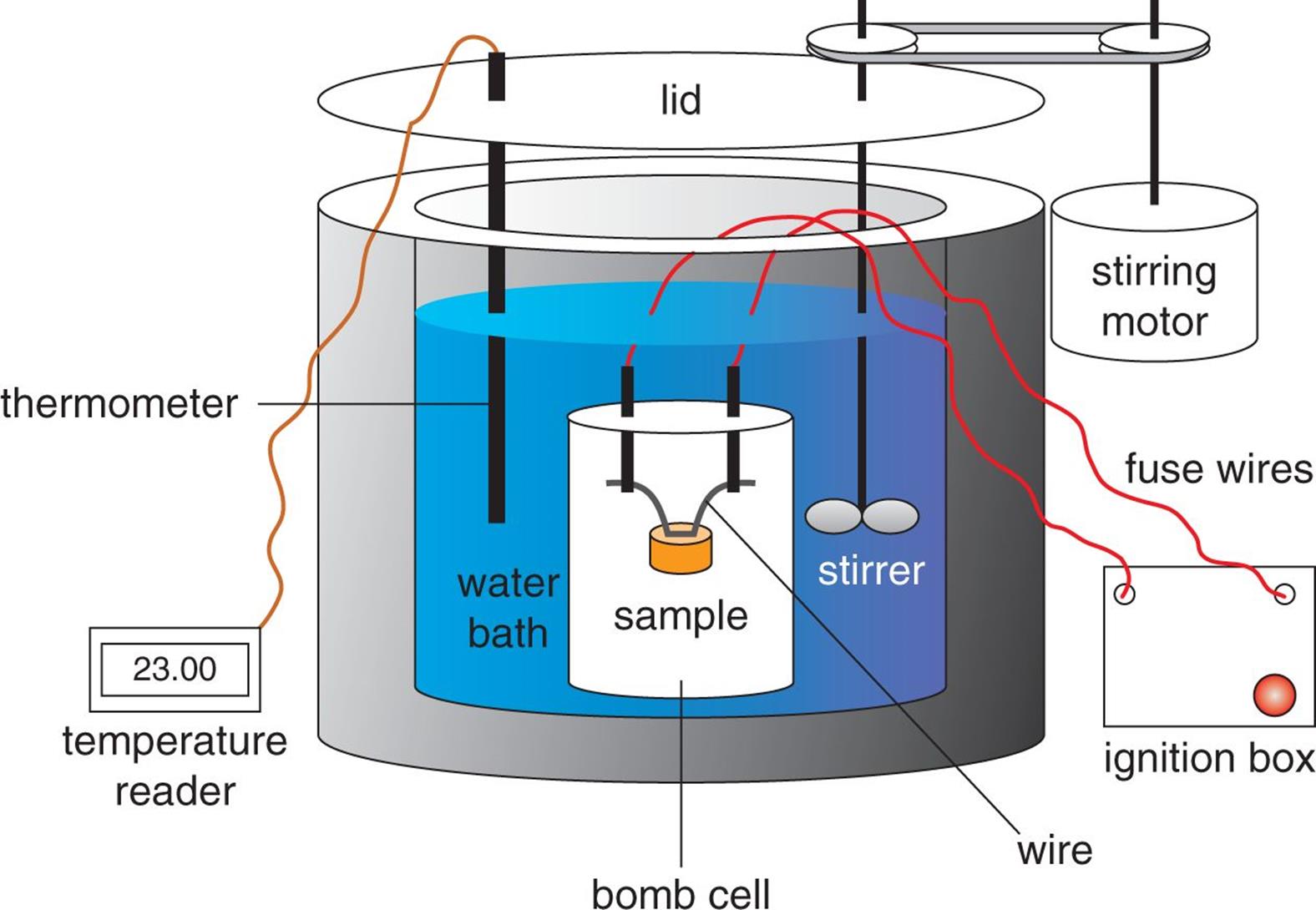
Figure 7.6. Diagram of a Bomb Calorimeter
Calorimeter Isolated System In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. To do so, the heat is exchanged with a calibrated object. Calorimeters are carefully insulated so that heat transfer in or out is negligible. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. Calorimetry is used to measure amounts of heat transferred to or from a substance. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter.
From www.researchgate.net
Opentype and closedtype calorimeters. The opentype calorimeter Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. To do so, the heat is exchanged with a calibrated object. Calorimeters are carefully insulated so that heat transfer in or out is negligible. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts. Calorimeter Isolated System.
From www.labmakelaar.eu
Malvern MicroCal Automated Differential Scanning Calorimeter Calorimeter Isolated System In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is an instrument. Calorimeter Isolated System.
From www.bartleby.com
Calorimetry bartleby Calorimeter Isolated System Calorimeters are carefully insulated so that heat transfer in or out is negligible. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. A calorimeter is an instrument that. Calorimeter Isolated System.
From www.medicalexpo.fr
Calorimètre par titration isothermique C 200 auto IKA de Calorimeter Isolated System In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters are carefully insulated so that heat transfer in or out. Calorimeter Isolated System.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6655927 Calorimeter Isolated System The function of the calorimeter depends on the conservation of energy in a closed, isolated system. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters are carefully insulated so that heat transfer in or out is negligible. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter.. Calorimeter Isolated System.
From www.medicalexpo.fr
Calorimètre par titration isothermique C 6000 1/10 IKA de laboratoire Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. Calorimetry is used to measure amounts of. Calorimeter Isolated System.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Isolated System In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Calorimetry is used to measure amounts. Calorimeter Isolated System.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimeter Isolated System In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. Calorimetry is used to measure amounts of heat transferred to or from a substance. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a. Calorimeter Isolated System.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Isolated System Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter. Calorimeter Isolated System.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Isolated System The function of the calorimeter depends on the conservation of energy in a closed, isolated system. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. Calorimeters are carefully insulated so that heat transfer in or out is negligible. Calorimetry. Calorimeter Isolated System.
From www.researchgate.net
Crosssection of the calorimeter. Download Scientific Diagram Calorimeter Isolated System Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. To do so, the heat is exchanged with a calibrated object. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Calorimetry is used to measure amounts of heat. Calorimeter Isolated System.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. The function of the calorimeter depends on the conservation. Calorimeter Isolated System.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter Isolated System Calorimeters are carefully insulated so that heat transfer in or out is negligible. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. Calorimetry is used to measure amounts of heat transferred to or from a substance. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter.. Calorimeter Isolated System.
From saylordotorg.github.io
Calorimetry Calorimeter Isolated System Calorimeters are carefully insulated so that heat transfer in or out is negligible. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. In practice, calorimetry depends on the. Calorimeter Isolated System.
From schoolbag.info
Figure 7.6. Diagram of a Bomb Calorimeter Calorimeter Isolated System To do so, the heat is exchanged with a calibrated object. Calorimeters are carefully insulated so that heat transfer in or out is negligible. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. The function of the calorimeter depends on the conservation of energy in a closed, isolated system.. Calorimeter Isolated System.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. Scientists measure the change in thermodynamic quantities in thermochemical equations. Calorimeter Isolated System.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5405762 Calorimeter Isolated System Calorimeters are carefully insulated so that heat transfer in or out is negligible. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. In practice, calorimetry depends on the use of an experimental system that approximates what. Calorimeter Isolated System.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. To do so, the heat is exchanged with a calibrated object. Calorimeters are carefully insulated so that heat transfer in or out is negligible. Calorimetry is used to measure amounts of heat transferred to or from a substance. In a. Calorimeter Isolated System.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimeter Isolated System To do so, the heat is exchanged with a calibrated object. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Calorimeters are carefully insulated so that heat transfer. Calorimeter Isolated System.
From ambrosiabaking.com
Coffee Cup Calorimeter A Simple And Inexpensive Way To Measure The Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there.. Calorimeter Isolated System.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Calorimeter Isolated System Calorimetry is used to measure amounts of heat transferred to or from a substance. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. To do so, the heat is exchanged with a calibrated object. In practice,. Calorimeter Isolated System.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download Calorimeter Isolated System Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. In practice, calorimetry depends on the use of an. Calorimeter Isolated System.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Isolated System Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. To do so, the heat is exchanged with a calibrated object. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. Calorimeters are carefully insulated so that heat transfer in or out is. Calorimeter Isolated System.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Calorimeter Isolated System In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. To do so,. Calorimeter Isolated System.
From byjus.com
The calorimeter is commonly made up of……..because it has……. Calorimeter Isolated System To do so, the heat is exchanged with a calibrated object. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Calorimetry is used to measure amounts of heat transferred to or from a substance. Scientists measure the change in thermodynamic quantities in thermochemical equations using. Calorimeter Isolated System.
From www.researchgate.net
Reaction calorimeter system used in the POL project. Download Calorimeter Isolated System Calorimeters are carefully insulated so that heat transfer in or out is negligible. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits. Calorimeter Isolated System.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Isolated System Calorimetry is used to measure amounts of heat transferred to or from a substance. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a. Calorimeter Isolated System.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Isolated System Calorimeters are carefully insulated so that heat transfer in or out is negligible. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. Scientists measure the change in thermodynamic quantities. Calorimeter Isolated System.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. To do so, the heat is exchanged with a calibrated object. The function of the calorimeter depends on the conservation of energy in. Calorimeter Isolated System.
From saylordotorg.github.io
Calorimetry Calorimeter Isolated System Calorimetry is used to measure amounts of heat transferred to or from a substance. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with its.. Calorimeter Isolated System.
From www.youtube.com
Thermochemistry Enthalpy and Coffee Cup Calorimeter. YouTube Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters are carefully insulated so that heat transfer in or out is negligible. In practice, calorimetry depends on the use of an experimental system that approximates. Calorimeter Isolated System.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Isolated System Calorimeters are carefully insulated so that heat transfer in or out is negligible. To do so, the heat is exchanged with a calibrated object. The function of the calorimeter depends on the conservation of energy in a closed, isolated system. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one. Calorimeter Isolated System.
From kaffee.50webs.com
Lab Calorimetry Calorimeter Isolated System A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a. Calorimeter Isolated System.
From ecampusontario.pressbooks.pub
3.5 Calorimétrie La Chimie Générale pour les GeeGees Calorimeter Isolated System In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters are carefully insulated so that heat transfer in or out is negligible. To do so, the heat is exchanged with a. Calorimeter Isolated System.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimeter Isolated System Calorimetry is used to measure amounts of heat transferred to or from a substance. Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter. In practice, calorimetry depends on the use of an experimental system that approximates what is termed an isolated system, one that permits no exchange of matter or energy with. Calorimeter Isolated System.