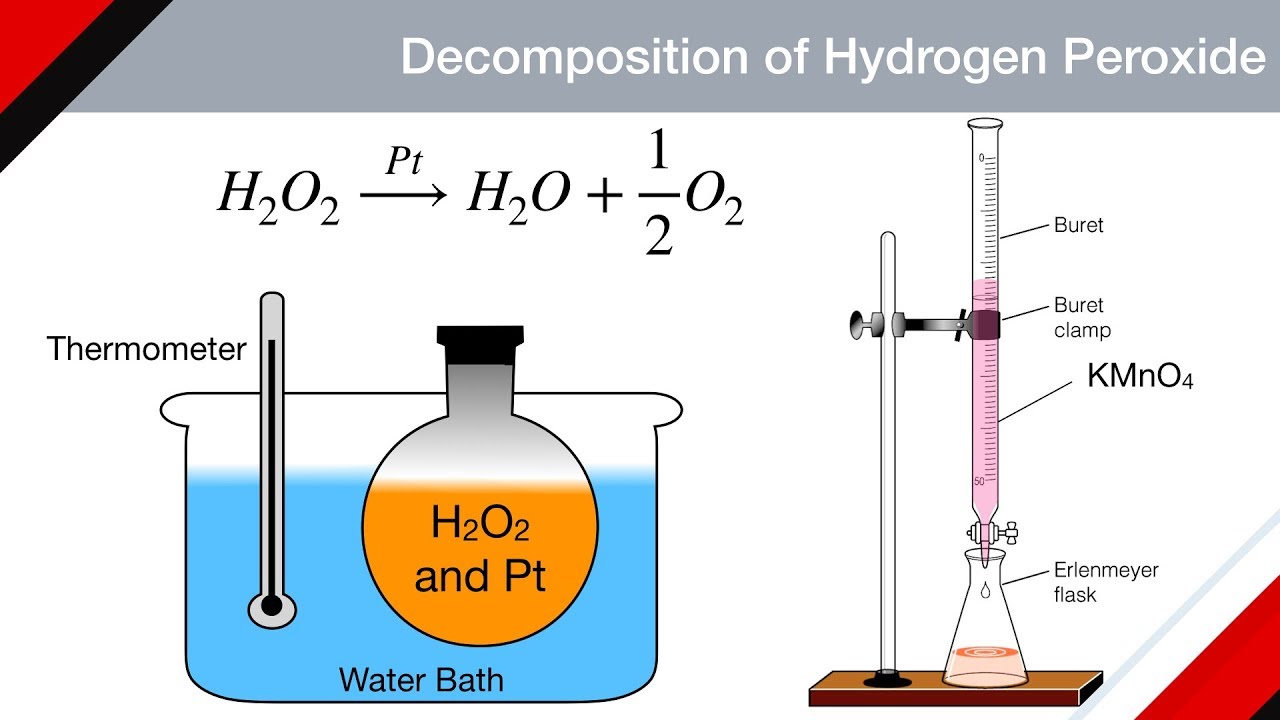
Metal Catalyst Hydrogen Peroxide Decomposition . Includes kit list and safety instructions. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalase enzyme (found in blood) lowers the activation energy to. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3).
from www.vrogue.co
The catalase enzyme (found in blood) lowers the activation energy to. Includes kit list and safety instructions. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3).
The Catalytic Of Hydrogen Peroxide Writ vrogue.co
Metal Catalyst Hydrogen Peroxide Decomposition The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. The catalase enzyme (found in blood) lowers the activation energy to. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Includes kit list and safety instructions. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). Platinum metal catalysts can lower the activation energy to about 49 kj/mol.
From www.slideserve.com
PPT Transition Elements&Catalysts PowerPoint Presentation, free Metal Catalyst Hydrogen Peroxide Decomposition The catalase enzyme (found in blood) lowers the activation energy to. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Collect a range of catalysts to explore the. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.vrogue.co
The Catalytic Of Hydrogen Peroxide Writ vrogue.co Metal Catalyst Hydrogen Peroxide Decomposition Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalase enzyme (found in blood) lowers the activation energy to. Platinum. Metal Catalyst Hydrogen Peroxide Decomposition.
From telegra.ph
Catalysis of hydrogen peroxide activation Telegraph Metal Catalyst Hydrogen Peroxide Decomposition This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. The catalytic decomposition of hydrogen peroxide allows the use of. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.vrogue.co
The Catalytic Of Hydrogen Peroxide Writ vrogue.co Metal Catalyst Hydrogen Peroxide Decomposition Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. The catalase enzyme (found in blood) lowers the activation energy to. The catalytic decomposition of hydrogen peroxide allows the use. Metal Catalyst Hydrogen Peroxide Decomposition.
From lessonlibrarypsilosis.z14.web.core.windows.net
Hydrogen Peroxide Experiment Metal Catalyst Hydrogen Peroxide Decomposition The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Includes kit list and safety instructions. Data on the. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.researchgate.net
(a) The conversions of the hydrogen peroxide catalyzed by Metal Catalyst Hydrogen Peroxide Decomposition Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalase enzyme (found in blood). Metal Catalyst Hydrogen Peroxide Decomposition.
From www.nagwa.com
Question Video Discerning the More Effective Catalyst for Hydrogen Metal Catalyst Hydrogen Peroxide Decomposition The catalase enzyme (found in blood) lowers the activation energy to. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). Includes kit list and safety instructions. Data on the decomposition of hydrogen peroxide on the. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.tessshebaylo.com
Hydrogen Peroxide Chemical Equation Tessshebaylo Metal Catalyst Hydrogen Peroxide Decomposition Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Includes kit list and safety instructions. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. The catalase enzyme (found in blood) lowers the activation energy to. This paper describes. Metal Catalyst Hydrogen Peroxide Decomposition.
From pixels.com
Hydrogen Peroxide Catalysis Photograph by Martyn F Metal Catalyst Hydrogen Peroxide Decomposition Includes kit list and safety instructions. The catalase enzyme (found in blood) lowers the activation energy to. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Platinum metal catalysts can. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.nagwa.com
Question Video Identifying the Balanced Chemical Equation for the Metal Catalyst Hydrogen Peroxide Decomposition Platinum metal catalysts can lower the activation energy to about 49 kj/mol. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalase enzyme (found in blood) lowers the activation energy to. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.cell.com
Design of framework catalysts for photocatalytic hydrogen Metal Catalyst Hydrogen Peroxide Decomposition The catalase enzyme (found in blood) lowers the activation energy to. Includes kit list and safety instructions. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.chegg.com
Solved 10) The of hydrogen peroxide can be Metal Catalyst Hydrogen Peroxide Decomposition Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates.. Metal Catalyst Hydrogen Peroxide Decomposition.
From pixels.com
Hydrogen Peroxide Catalysis Photograph by Martyn F Metal Catalyst Hydrogen Peroxide Decomposition Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Includes kit list and safety instructions. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Metal Catalyst Hydrogen Peroxide Decomposition Includes kit list and safety instructions. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalytic decomposition of hydrogen peroxide. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.numerade.com
SOLVED The of hydrogen peroxide, H2O2, is catalyzed by Metal Catalyst Hydrogen Peroxide Decomposition The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo,. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.pnas.org
Active sites and mechanisms for H2O2 over Pd catalysts PNAS Metal Catalyst Hydrogen Peroxide Decomposition Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. The catalase enzyme. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Metal Catalyst Hydrogen Peroxide Decomposition Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Data on the decomposition of hydrogen peroxide on the. Metal Catalyst Hydrogen Peroxide Decomposition.
From jamirghopallen.blogspot.com
Describe Using a Chemical Equation the Breakdown of Hydrogen Peroxide Metal Catalyst Hydrogen Peroxide Decomposition Includes kit list and safety instructions. The catalase enzyme (found in blood) lowers the activation energy to. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Platinum metal. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.researchgate.net
Schematic of hydrogen peroxide on the prepared catalyst Metal Catalyst Hydrogen Peroxide Decomposition This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalase enzyme (found in blood) lowers the activation energy to. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Platinum metal catalysts can lower the activation energy. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.pnas.org
Active sites and mechanisms for H2O2 over Pd catalysts PNAS Metal Catalyst Hydrogen Peroxide Decomposition The catalase enzyme (found in blood) lowers the activation energy to. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph <. Metal Catalyst Hydrogen Peroxide Decomposition.
From reubengokegoodwin.blogspot.com
of Hydrogen Peroxide Equation Metal Catalyst Hydrogen Peroxide Decomposition The catalase enzyme (found in blood) lowers the activation energy to. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Collect a range of catalysts to explore the decomposition. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.youtube.com
Effect of Various Catalysts on Hydrogen Peroxide GCSE Chemistry YouTube Metal Catalyst Hydrogen Peroxide Decomposition Platinum metal catalysts can lower the activation energy to about 49 kj/mol. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Includes kit list and safety instructions. Collect. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Metal Catalyst Hydrogen Peroxide Decomposition The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Includes kit list and safety instructions. Collect a range of catalysts to explore. Metal Catalyst Hydrogen Peroxide Decomposition.
From worksheetlistvi.z21.web.core.windows.net
Concentration Hydrogen Peroxide Experiment Metal Catalyst Hydrogen Peroxide Decomposition The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalase enzyme (found in blood) lowers the activation energy to. Data on the decomposition of hydrogen peroxide on. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.sciencephoto.com
Catalytic of Hydrogen Peroxide Stock Image C030/7308 Metal Catalyst Hydrogen Peroxide Decomposition Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalase enzyme (found in blood) lowers the activation energy to. Data on the decomposition of hydrogen peroxide on the ag 2. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.mdpi.com
Catalysts Free FullText Looking for the “Dream Catalyst” for Metal Catalyst Hydrogen Peroxide Decomposition The catalase enzyme (found in blood) lowers the activation energy to. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. This paper. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.scribd.com
Catalysts for the of Hydrogen Peroxide Catalysis Metal Catalyst Hydrogen Peroxide Decomposition Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Includes kit list and safety instructions. Platinum metal catalysts can lower the. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.sciencephoto.com
Catalytic of Hydrogen Peroxide Stock Image C030/7305 Metal Catalyst Hydrogen Peroxide Decomposition Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalytic decomposition of hydrogen peroxide. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.researchgate.net
Possible mechanism of hydrogen peroxide catalyzed by Metal Catalyst Hydrogen Peroxide Decomposition Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalase enzyme (found in blood) lowers the activation energy to. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. The catalytic decomposition of hydrogen peroxide allows the use. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.researchgate.net
Scheme II Reaction scheme for the catalytic of hydrogen Metal Catalyst Hydrogen Peroxide Decomposition Includes kit list and safety instructions. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. The catalase enzyme (found in blood) lowers the activation energy to. Collect a. Metal Catalyst Hydrogen Peroxide Decomposition.
From fineartamerica.com
Hydrogen Peroxide Catalysis Photograph by Martyn F. Chillmaid Metal Catalyst Hydrogen Peroxide Decomposition The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. This paper describes a kinetic model for the decomposition. Metal Catalyst Hydrogen Peroxide Decomposition.
From hager-engineering.com
High performance catalysts Hydrogen Peroxide Catalysts Ozone Metal Catalyst Hydrogen Peroxide Decomposition Includes kit list and safety instructions. This paper describes a kinetic model for the decomposition of hydrogen peroxide by ferric ion in homogeneous aqueous solution (ph < 3). Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. The catalase enzyme (found. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.bartleby.com
Answered The of hydrogen peroxide… bartleby Metal Catalyst Hydrogen Peroxide Decomposition The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. The catalase enzyme (found in blood) lowers the activation energy to. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. Includes kit. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.slideserve.com
PPT of Hydrogen Peroxide PowerPoint Presentation, free Metal Catalyst Hydrogen Peroxide Decomposition Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and. Metal Catalyst Hydrogen Peroxide Decomposition.
From www.flinnsci.com
Catalytic of Hydrogen Peroxide Flinn Scientific Metal Catalyst Hydrogen Peroxide Decomposition Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Data on the decomposition of hydrogen peroxide on the ag 2 o, ruo 2, mno 2, mn 2 o 3, pbo, and v 2 o 5 active phases. The catalase enzyme (found in blood) lowers the activation energy to. This. Metal Catalyst Hydrogen Peroxide Decomposition.