Boiling Water Steam Temperature . The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. Steam (actually, water vapor) above a rapidly boiling can be hotter than the average temperature of the liquid because the situation. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. In fact, at the microscopic level, there may be cooler regions of boiling water. Note that once your open pot of water hits the. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone. These vaporised molecules, possessing same. You can crank the heat as high as you like. At 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 oc.
from socratic.org
So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. In fact, at the microscopic level, there may be cooler regions of boiling water. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. Note that once your open pot of water hits the. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. You can crank the heat as high as you like. Steam (actually, water vapor) above a rapidly boiling can be hotter than the average temperature of the liquid because the situation.
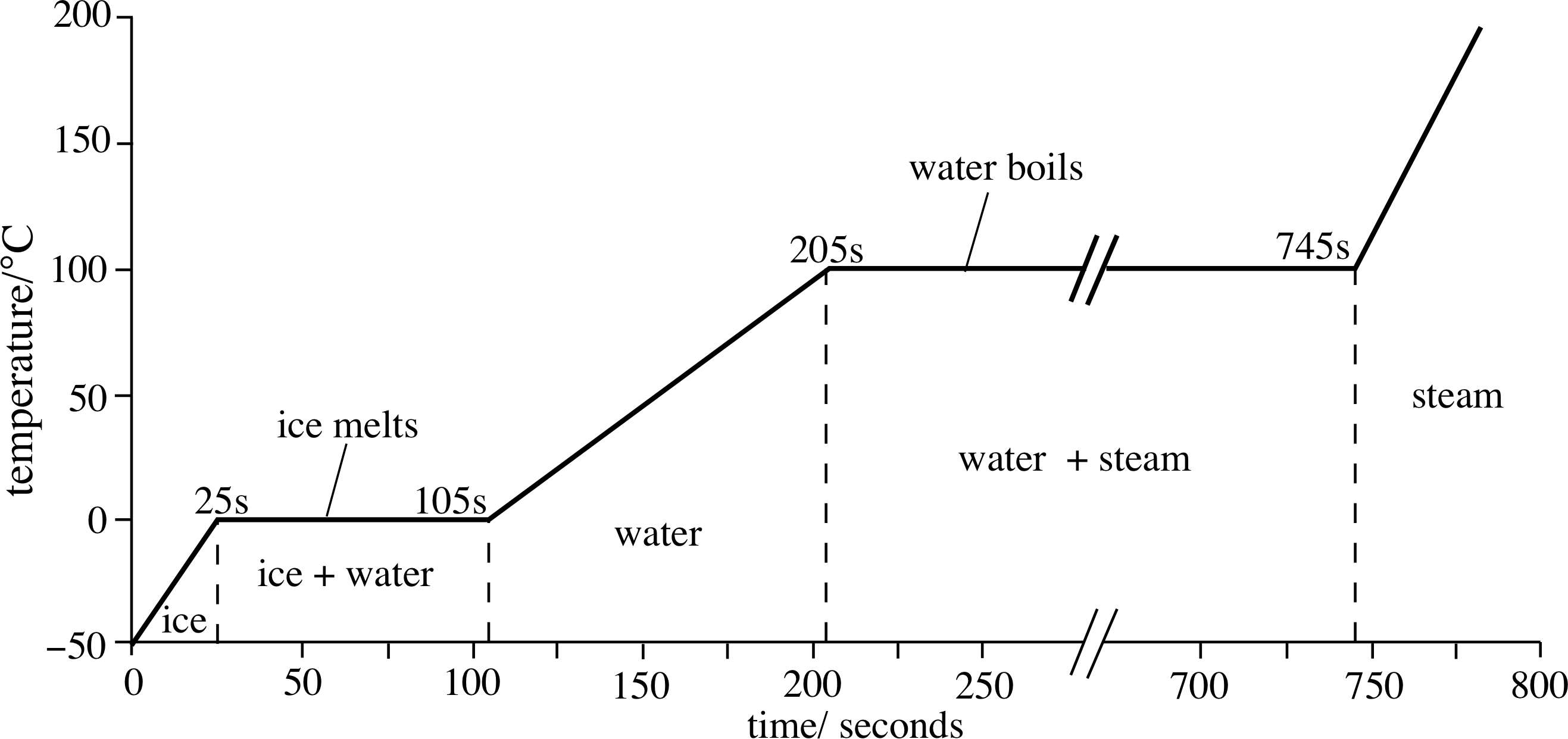
What is the profile of the graph of temperature versus time, when water
Boiling Water Steam Temperature When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. You can crank the heat as high as you like. At 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 oc. So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone. These vaporised molecules, possessing same. Steam (actually, water vapor) above a rapidly boiling can be hotter than the average temperature of the liquid because the situation. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. In fact, at the microscopic level, there may be cooler regions of boiling water. Note that once your open pot of water hits the. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat.
From slideplayer.com
Solids, Liquids and Gases ppt download Boiling Water Steam Temperature More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. Steam (actually, water vapor) above a rapidly boiling can be hotter than the average temperature of the liquid because the situation. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The 'boiling. Boiling Water Steam Temperature.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Boiling Water Steam Temperature You can crank the heat as high as you like. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. Note that once your open pot of water hits the. The 'boiling point'. Boiling Water Steam Temperature.
From mavink.com
Water Boiling Pressure Chart Boiling Water Steam Temperature The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. You can crank the heat as high as you like. So once the water hits. Boiling Water Steam Temperature.
From recipepes.com
steam temperature chart Boiling Water Steam Temperature Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. These vaporised molecules, possessing same. So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating. Boiling Water Steam Temperature.
From www.shutterstock.com
2,362 Boiling Water Experiment RoyaltyFree Images, Stock Photos Boiling Water Steam Temperature You can crank the heat as high as you like. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. Note that once your open pot of water hits the. At. Boiling Water Steam Temperature.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Boiling Water Steam Temperature When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the. Boiling Water Steam Temperature.
From klaoasptt.blob.core.windows.net
Does Boiling Water Leave Scars at Diane Pierre blog Boiling Water Steam Temperature When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. You can crank the heat as high as you like. In fact, at the microscopic level, there may be cooler regions of boiling. Boiling Water Steam Temperature.
From www.foodabovegold.com
Steaming Cooking Methods 101 Food Above Gold Boiling Water Steam Temperature Note that once your open pot of water hits the. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. The temperature. Boiling Water Steam Temperature.
From aitchaeriesh.blogspot.com
33+ calculate heat absorbed by water AitchAeriesh Boiling Water Steam Temperature You can crank the heat as high as you like. Note that once your open pot of water hits the. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. When vapor bubbles form near a heat source, like at the bottom of a. Boiling Water Steam Temperature.
From vandewaterbooks.com
What Temperature Does Water Boil At? Boiling Point & Elevation Vande Boiling Water Steam Temperature Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. You can crank the heat as high as you like. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The temperature of the boiling water and saturated steam within the same system. Boiling Water Steam Temperature.
From www.quora.com
What makes water, ice, and steam different from one another? Quora Boiling Water Steam Temperature So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. The water may boil more vigorously and convert into steam. Boiling Water Steam Temperature.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Boiling Water Steam Temperature More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the. Boiling Water Steam Temperature.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning Boiling Water Steam Temperature The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. These vaporised molecules, possessing same. In fact, at the microscopic level, there may be cooler regions of boiling water. Steam (actually, water vapor) above a rapidly boiling can be hotter than the. Boiling Water Steam Temperature.
From sciencenotes.org
How to Boil Water at Room Temperature Boiling Water Steam Temperature So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. In fact, at the microscopic level, there may. Boiling Water Steam Temperature.
From www.sciencelearn.org.nz
A pot of boiling water — Science Learning Hub Boiling Water Steam Temperature The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Note that once your open pot of water hits the. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from. Boiling Water Steam Temperature.
From www.teachoo.com
What produces more severe burns, boiling water or steam? (Teachoo) Boiling Water Steam Temperature The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. In fact, at the microscopic level, there may be cooler regions of boiling water. These vaporised molecules, possessing same. Steam (actually, water vapor) above a rapidly boiling can be hotter than the. Boiling Water Steam Temperature.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Boiling Water Steam Temperature Note that once your open pot of water hits the. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. At 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 oc. More heat energy is required to raise its temperature. Boiling Water Steam Temperature.
From www.resortrehab.com
マサル Water,Steam,and Aque 9784621075968ぐるぐる王国DS ヤフー店 通販 マツバヤシ Boiling Water Steam Temperature The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. Steam (actually, water vapor) above a rapidly boiling can be hotter than the average temperature of the liquid because the situation. So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone.. Boiling Water Steam Temperature.
From www.answerthehome.com
At What Temperature Does Water Steam? Boiling Water Steam Temperature The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. In fact, at the microscopic level, there may be cooler regions of boiling water. These vaporised molecules, possessing same. You can crank the heat as high as you like. More heat energy is required. Boiling Water Steam Temperature.
From www.boiler-planning.com
Steam types Bosch steam boiler planning Commercial & Industrial Boiling Water Steam Temperature When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. These vaporised molecules, possessing same. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. More. Boiling Water Steam Temperature.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Boiling Water Steam Temperature More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes. Boiling Water Steam Temperature.
From www.researchgate.net
Densities of saturated water and steam vapour in the vicinity of the Boiling Water Steam Temperature The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. These vaporised molecules, possessing same. You can crank the heat as high as you like. Note that once your open pot of water hits the. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed. Boiling Water Steam Temperature.
From queenwins.en.made-in-china.com
Display Temperature Water Boiler Steam and Boiling Water Machine Boiling Water Steam Temperature When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. Steam (actually, water vapor) above a rapidly boiling can be hotter than the average temperature of the liquid because the situation. The water may boil more vigorously and convert into steam more quickly, but it won’t. Boiling Water Steam Temperature.
From www.thekitchn.com
7 Surprising Ways to Use Boiling Water to Clean The Kitchn Boiling Water Steam Temperature You can crank the heat as high as you like. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. Steam (actually, water vapor) above a rapidly boiling can. Boiling Water Steam Temperature.
From www.youtube.com
What causes Severe Burns Steam or Boiling water ? YouTube Boiling Water Steam Temperature More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. Steam (actually, water. Boiling Water Steam Temperature.
From www.youtube.com
At What Temperature Does Water Steam? Know Now YouTube Boiling Water Steam Temperature The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. At 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 oc. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. Boiling starts. Boiling Water Steam Temperature.
From www.dreamstime.com
Water Boiling in a Pan. Hot Steam from the Pan. High Temperature Water Boiling Water Steam Temperature More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. In fact, at the microscopic level, there may be cooler regions of boiling water. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. The 'boiling point' of water is the temperature at which steam and liquid. Boiling Water Steam Temperature.
From www.boiler-planning.com
Presión y temperatura Bosch Planificación de calderas de vapor Boiling Water Steam Temperature At 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 oc. These vaporised molecules, possessing same. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. The 'boiling point' of water is the temperature at which steam and liquid exist. Boiling Water Steam Temperature.
From www.valvesonline.com.au
Steam Tables Pressure vs Temperature Boiling Water Steam Temperature The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. You can crank the heat as high as you like. In fact, at the microscopic level, there may be cooler regions of boiling water. When vapor bubbles form near a heat source,. Boiling Water Steam Temperature.
From www.youtube.com
Why does steam cause more severe burns than boiling water? science Boiling Water Steam Temperature The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. Steam (actually, water vapor) above a rapidly boiling can be hotter than the average temperature of the liquid because the situation. At 7 bar g (absolute 8 bar) the saturation temperature of water is. Boiling Water Steam Temperature.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Boiling Water Steam Temperature The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. Note that once your open pot of water hits the. In fact, at the microscopic level, there may be cooler regions of boiling water. At 7 bar g (absolute 8 bar) the saturation temperature. Boiling Water Steam Temperature.
From socratic.org
What is the profile of the graph of temperature versus time, when water Boiling Water Steam Temperature The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater. In fact, at the microscopic level, there may be cooler regions of boiling water. You can crank the heat as high as you like. Note that once your open pot of water hits the.. Boiling Water Steam Temperature.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent Boiling Water Steam Temperature These vaporised molecules, possessing same. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. So once the water hits 212 f (=100 c = 373 k), it just keeps evaporating til all the liquid is gone. Note that once your open. Boiling Water Steam Temperature.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent Boiling Water Steam Temperature Note that once your open pot of water hits the. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed when the. At 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 oc. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. When vapor. Boiling Water Steam Temperature.
From www.scienceabc.com
Are Evaporation And Boiling The Same? » ScienceABC Boiling Water Steam Temperature At 7 bar g (absolute 8 bar) the saturation temperature of water is 170.42 oc. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per. Boiling Water Steam Temperature.