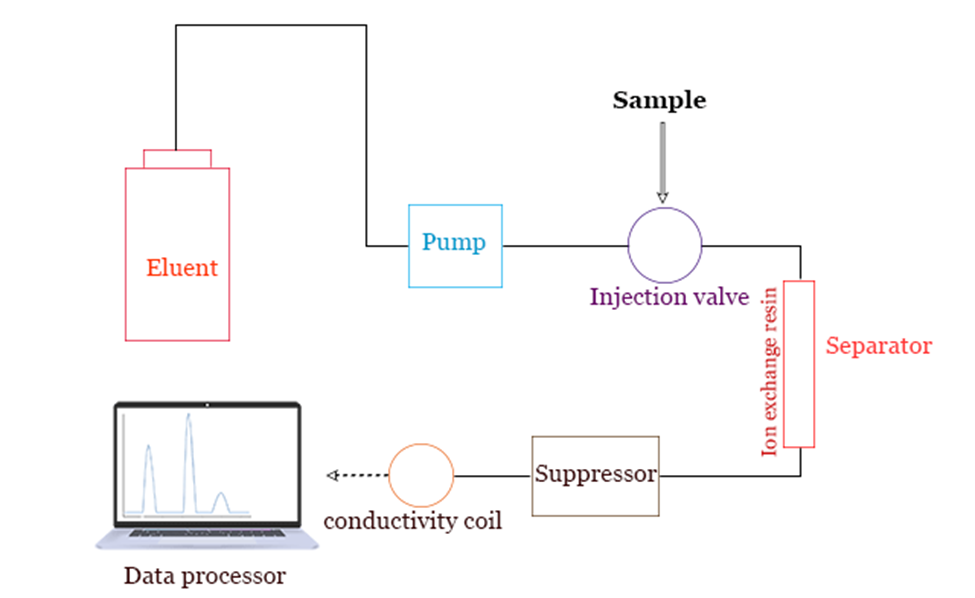
Ion Exchange Chromatography Buffers . Start buffer including 1 m nacl, ph 6.0. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange It is a versatile and. A number of important factors influences the selection of mobile phase including buffer charge, buffer.
from scienceinfo.com
It is a versatile and. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. A number of important factors influences the selection of mobile phase including buffer charge, buffer. Start buffer including 1 m nacl, ph 6.0.
Ion Exchange Chromatography Principle, Types, Procedure, Applications
Ion Exchange Chromatography Buffers Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Start buffer including 1 m nacl, ph 6.0. It is a versatile and. A number of important factors influences the selection of mobile phase including buffer charge, buffer. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge.
From www.semanticscholar.org
[PDF] Chapter 2 IonExchange Chromatography and Its Applications Ion Exchange Chromatography Buffers Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. It is a versatile and. A number of important factors. Ion Exchange Chromatography Buffers.
From www.intechopen.com
IonExchange Chromatography and Its Applications IntechOpen Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Ionexchange chromatography of raw whey protein WPPEI HR 5/5. Eluting Ion Exchange Chromatography Buffers Start buffer including 1 m nacl, ph 6.0. A number of important factors influences the selection of mobile phase including buffer charge, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange When choosing a buffer. Ion Exchange Chromatography Buffers.
From www.slideserve.com
PPT BY1101 Introduction to Molecular and Cellular Biology PowerPoint Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange When choosing a buffer system for ion exchange chromatography, multiple factors should. Ion Exchange Chromatography Buffers.
From scienceinfo.com
Ion Exchange Chromatography Principle, Types, Procedure, Applications Ion Exchange Chromatography Buffers Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange It is a versatile and. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. When. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Cation exchange chromatography Elution buffer 0.05 M TrisHCl ( pH Ion Exchange Chromatography Buffers Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Start buffer including 1 m nacl, ph 6.0. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for. Ion Exchange Chromatography Buffers.
From microbenotes.com
Ion Exchange Chromatography Principle, Parts, Steps, Uses Ion Exchange Chromatography Buffers Start buffer including 1 m nacl, ph 6.0. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. A number of important factors influences the selection of mobile phase including buffer charge, buffer. It is a versatile and. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Ion Exchange Chromatography of BMP2 E94Plk reveals presence of dimeric Ion Exchange Chromatography Buffers It is a versatile and. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1),. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Ion Exchange Chromatography allows a clear separation of BMP2 E83Plk Ion Exchange Chromatography Buffers Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Start buffer including 1 m nacl, ph 6.0. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Schematic illustration of ionexchange chromatography. The Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Start buffer including 1 m nacl, ph 6.0. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph,. Ion Exchange Chromatography Buffers.
From microbiologynotes.org
Ion Exchange chromatography Principle, Technique and Application Ion Exchange Chromatography Buffers When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. It is a versatile and. A number of important factors influences the selection of mobile phase including buffer charge, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while. Ion Exchange Chromatography Buffers.
From www.slideserve.com
PPT Ion Exchange Chromatography PowerPoint Presentation, free Ion Exchange Chromatography Buffers Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange A number of important factors influences the selection of mobile phase including buffer charge, buffer. It is a versatile and. Start buffer including 1 m nacl, ph. Ion Exchange Chromatography Buffers.
From kh.aquaenergyexpo.com
Ion Exchange Chromatography AquaEnergy Expo Knowledge Hub Ion Exchange Chromatography Buffers Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Start buffer including 1 m nacl,. Ion Exchange Chromatography Buffers.
From www.slideserve.com
PPT Ion exchange chromatography PowerPoint Presentation, free Ion Exchange Chromatography Buffers Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Start buffer including 1 m nacl, ph 6.0. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for. Ion Exchange Chromatography Buffers.
From gbu-presnenskij.ru
Gel Filtration Chromatography An Overview ScienceDirect, 50 OFF Ion Exchange Chromatography Buffers Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. A number of important factors influences the selection of mobile phase including buffer charge, buffer. It is a versatile and. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2). Ion Exchange Chromatography Buffers.
From scienceinfo.com
Ion Exchange Chromatography Principle, Types, Procedure, Applications Ion Exchange Chromatography Buffers It is a versatile and. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. A number of important factors influences the selection of mobile phase including buffer charge, buffer. Start buffer including 1 m nacl, ph 6.0. Typical buffers used as mobile phases for cation exchange chromatography include. Ion Exchange Chromatography Buffers.
From ar.inspiredpencil.com
Ion Exchange Chromatography Ion Exchange Chromatography Buffers When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Start buffer including 1 m nacl, ph 6.0. It is a versatile and. A number of important factors influences the selection of mobile phase including buffer charge, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6),. Ion Exchange Chromatography Buffers.
From www.whdsbio.cn
What buffers are commonly used in exchange chromatography?_Hubei New Ion Exchange Chromatography Buffers When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Start buffer including 1 m nacl, ph 6.0. It is. Ion Exchange Chromatography Buffers.
From info.gbiosciences.com
Gel Filtration and Its Role in Desalting and Buffer Exchange Ion Exchange Chromatography Buffers Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Start buffer including 1 m nacl, ph 6.0. It is a versatile and. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Typical elution profi les and SDSPAGE analysis of DEAE ionexchange Ion Exchange Chromatography Buffers Start buffer including 1 m nacl, ph 6.0. A number of important factors influences the selection of mobile phase including buffer charge, buffer. It is a versatile and. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion. Ion Exchange Chromatography Buffers.
From www.cytivalifesciences.com.cn
Hydrophobic Interaction Chromatography Cytiva Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. It is a versatile and. Typical buffers. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Ionexchange chromatography of proteins in the desalted P80 fraction Ion Exchange Chromatography Buffers Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Start buffer including 1 m nacl,. Ion Exchange Chromatography Buffers.
From go.labmanager.com
Ion Exchange Chromatography Buffers Lab Manager Ion Exchange Chromatography Buffers When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1),. Ion Exchange Chromatography Buffers.
From www.slideshare.net
ION EXCHANGE CHROMATOGRAPHY Ion Exchange Chromatography Buffers Start buffer including 1 m nacl, ph 6.0. A number of important factors influences the selection of mobile phase including buffer charge, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes. Ion Exchange Chromatography Buffers.
From www.lanlangcorp.com
How does ion exchange chromatography resin work? FAQ Taiyuan Ion Exchange Chromatography Buffers Start buffer including 1 m nacl, ph 6.0. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. It is a versatile and. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are. Ion Exchange Chromatography Buffers.
From www.slideserve.com
PPT ION EXCHANGE CHROMATOGRAPHY PowerPoint Presentation, free Ion Exchange Chromatography Buffers Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Start buffer including 1 m nacl, ph 6.0. A number of important factors influences the selection of mobile phase including buffer charge, buffer. When choosing a buffer. Ion Exchange Chromatography Buffers.
From www.researchgate.net
Schematic illustration of ionexchange chromatography. The Ion Exchange Chromatography Buffers Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Start buffer including 1 m nacl,. Ion Exchange Chromatography Buffers.
From jayceabbfritz.blogspot.com
Basic Principle of Ion Exchange Chromatography JayceabbFritz Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. It is a versatile and. Start buffer including 1 m nacl, ph 6.0. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6),. Ion Exchange Chromatography Buffers.
From www.mdpi.com
Biosensors Free FullText Rapid and Simple Buffer Exchange Using Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. It is a versatile and. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Start buffer including 1 m nacl, ph 6.0. Typical buffers used as mobile phases for cation exchange chromatography include. Ion Exchange Chromatography Buffers.
From ar.inspiredpencil.com
Ion Exchange Chromatography Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. Start buffer including 1 m nacl, ph 6.0. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. It is a versatile and. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on. Ion Exchange Chromatography Buffers.
From medium.com
Applications of ion exchange chromatography Study Chemistry Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange It is a versatile and. When choosing a buffer system for ion. Ion Exchange Chromatography Buffers.
From chemistnotes.com
Ion Exchange Chromatography,Defination, principle and advantages Ion Exchange Chromatography Buffers A number of important factors influences the selection of mobile phase including buffer charge, buffer. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Start buffer including 1 m nacl, ph 6.0. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph,. Ion Exchange Chromatography Buffers.
From www.gbiosciences.com
The rAmylase Project Using Ion Exchange chromatography to separate Ion Exchange Chromatography Buffers Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. A number of important factors influences the selection of mobile phase including buffer charge, buffer. Start buffer including 1 m nacl, ph 6.0. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph,. Ion Exchange Chromatography Buffers.
From www.biorender.com
Anion and Cation Exchange Chromatography BioRender Science Templates Ion Exchange Chromatography Buffers Start buffer including 1 m nacl, ph 6.0. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6), mes (6.1), phosphate (7.2) or tris (8.1), while buffers that are used as mobile phases for anion exchange It is a versatile and. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups. Ion Exchange Chromatography Buffers.
From www.numerade.com
SOLVEDIon Exchange Chromatography Answer the following questions Plot Ion Exchange Chromatography Buffers Start buffer including 1 m nacl, ph 6.0. It is a versatile and. A number of important factors influences the selection of mobile phase including buffer charge, buffer. When choosing a buffer system for ion exchange chromatography, multiple factors should be considered, including ph, buffer. Typical buffers used as mobile phases for cation exchange chromatography include formate (3.8), acetate (4.6),. Ion Exchange Chromatography Buffers.