Cap Requirements For Instrument Validation . download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments.
from www.sampletemplate.my.id
learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments.
Validation Certificate Template Sampletemplate.my.id
Cap Requirements For Instrument Validation learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. with this guideline update, validation requirements for all predictive markers have been harmonized. as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical.
From exotijeyh.blob.core.windows.net
Cap Requirements For Competency Assessment at Sharon Mart blog Cap Requirements For Instrument Validation learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. learn how to comply with cap. Cap Requirements For Instrument Validation.
From www.studocu.com
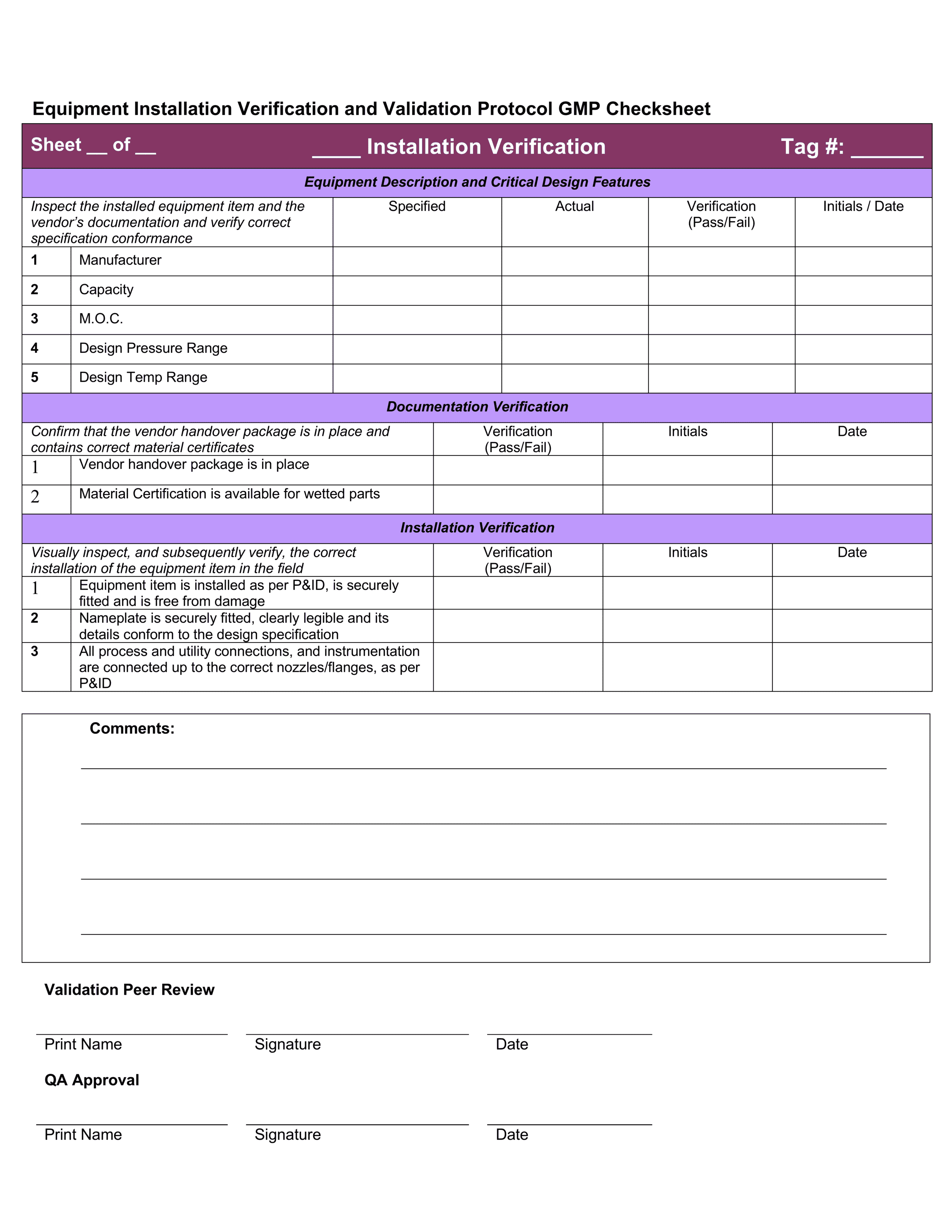
Instrument Validation FORM Validation FORM INSTRUMENT VALIDATION FORM Cap Requirements For Instrument Validation as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. download the all common checklist for. Cap Requirements For Instrument Validation.
From www.studocu.com
Validation of Research Instrument Validation of the Research Cap Requirements For Instrument Validation learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. with this guideline update, validation requirements for all predictive markers have been harmonized. as specified in the cap lap chemistry and toxicology. Cap Requirements For Instrument Validation.
From www.slideserve.com
PPT Understanding HB 2643 Sections 14 & 15 PowerPoint Presentation Cap Requirements For Instrument Validation find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\. Cap Requirements For Instrument Validation.
From www.presentationeze.com
Medical Device Validation Full Details PresentationEZE Cap Requirements For Instrument Validation with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. as specified in the cap lap chemistry and toxicology checklist, laboratories. Cap Requirements For Instrument Validation.
From www.sampletemplate.my.id
Validation Certificate Template Sampletemplate.my.id Cap Requirements For Instrument Validation learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. download the all common checklist. Cap Requirements For Instrument Validation.
From www.researchgate.net
(PDF) Requirements Validation Techniques An Empirical Study Cap Requirements For Instrument Validation find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. download the all common checklist for cap accreditation programs, which contains all. Cap Requirements For Instrument Validation.
From www.scribd.com
Instrument Validation Form PDF Cap Requirements For Instrument Validation download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. learn how to comply with cap requirements for analytical validation. Cap Requirements For Instrument Validation.
From www.scribd.com
Survey Instrument Validation Scale PDF Cap Requirements For Instrument Validation as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. download the all common checklist. Cap Requirements For Instrument Validation.
From www.scribd.com
Corrective Action Plan (CAP) Implementation Evaluation Checklist Cap Requirements For Instrument Validation learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. with this guideline update, validation requirements for all predictive markers have been harmonized. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. find the cap accreditation program requirements for various subdisciplines of. Cap Requirements For Instrument Validation.
From www.slideshare.net
Validation Master Plan Cap Requirements For Instrument Validation find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the. Cap Requirements For Instrument Validation.
From www.studypool.com
SOLUTION Survey instrument validation rating scale Studypool Cap Requirements For Instrument Validation with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. find the cap accreditation program requirements for various. Cap Requirements For Instrument Validation.
From www.slideshare.net
Clinical labmethod validation.ppt Cap Requirements For Instrument Validation learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. with this guideline update, validation requirements for all predictive markers have been harmonized. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to verify the accuracy and range of your laboratory. Cap Requirements For Instrument Validation.
From www.slideserve.com
PPT Individual Advising Plan (IAP) PowerPoint Presentation, free Cap Requirements For Instrument Validation with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v. Cap Requirements For Instrument Validation.
From www.scribd.com
Instrument Validation and Inspection Methods PDF Verification And Cap Requirements For Instrument Validation with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. as specified in the cap lap chemistry and toxicology checklist,. Cap Requirements For Instrument Validation.
From www.slideserve.com
PPT Understanding Nonprofits and the National Forest Foundation (NFF Cap Requirements For Instrument Validation find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. as specified in the cap lap chemistry and. Cap Requirements For Instrument Validation.
From slidetodoc.com
CAP Accreditation and Checklists Update California Clinical Laboratory Cap Requirements For Instrument Validation 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. with this guideline update, validation requirements for all predictive markers have been harmonized. find the cap accreditation program requirements for various subdisciplines of pathology. Cap Requirements For Instrument Validation.
From dxonxarcb.blob.core.windows.net
Model Validation Checklist at Lois Witherspoon blog Cap Requirements For Instrument Validation learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v. Cap Requirements For Instrument Validation.
From www.studocu.com
Survey Instrument Validation Rating Scale 2 SURVEY INSTRUMENT Cap Requirements For Instrument Validation as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. with this guideline update, validation requirements for all predictive markers have been harmonized. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. learn how to comply with cap requirements for analytical validation. Cap Requirements For Instrument Validation.
From www.slideserve.com
PPT Method Validation Studies It’s a Jungle out There! PowerPoint Cap Requirements For Instrument Validation with this guideline update, validation requirements for all predictive markers have been harmonized. download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to verify the accuracy and range of your. Cap Requirements For Instrument Validation.
From www.youtube.com
CapCut System Requirements Cap Cut Requirements Minimum Cap Requirements For Instrument Validation as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. find the. Cap Requirements For Instrument Validation.
From www.scribd.com
Certificate of Instrument Validation JJCV PDF Cap Requirements For Instrument Validation find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. with this guideline update, validation requirements for all predictive markers have been harmonized. download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. as specified in the cap lap chemistry and toxicology checklist, laboratories are required. Cap Requirements For Instrument Validation.
From www.scribd.com
Certificate of Instrument Validation PDF Cap Requirements For Instrument Validation learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. with this guideline update, validation requirements for all predictive markers have been harmonized. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to meet clia and lap requirements for calibration. Cap Requirements For Instrument Validation.
From www.slideserve.com
PPT E pluribus unum Regulatory Perspectives on Validating Moderately Cap Requirements For Instrument Validation learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to meet clia. Cap Requirements For Instrument Validation.
From www.scribd.com
CAP Accreditation Requirements For Validation of Laboratory Tests PDF Cap Requirements For Instrument Validation download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. with this guideline update, validation requirements for all predictive. Cap Requirements For Instrument Validation.
From www.slideshare.net
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION Cap Requirements For Instrument Validation find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. learn how to comply with cap requirements for analytical validation or verification of nonwaived. Cap Requirements For Instrument Validation.
From gioenupdv.blob.core.windows.net
Instrument Validation Report at Donald Dement blog Cap Requirements For Instrument Validation learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. learn how. Cap Requirements For Instrument Validation.
From www.studocu.com
Survey Instrument Validation for Research Bachelor of Science in Cap Requirements For Instrument Validation learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. with this guideline update, validation requirements for. Cap Requirements For Instrument Validation.
From exoocgzyu.blob.core.windows.net
Cap Sop Requirements at Jason Oldham blog Cap Requirements For Instrument Validation download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. learn how. Cap Requirements For Instrument Validation.
From www.slideserve.com
PPT Instrument, Spacecraft, and Launch Overview PowerPoint Cap Requirements For Instrument Validation as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. find the cap accreditation program requirements. Cap Requirements For Instrument Validation.
From issuu.com
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd Issuu Cap Requirements For Instrument Validation learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v. Cap Requirements For Instrument Validation.
From www.scribd.com
RESEARCH INSTRUMENT VALIDATION SHEET PDF Cap Requirements For Instrument Validation 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. with this guideline update, validation. Cap Requirements For Instrument Validation.
From www.researchgate.net
(PDF) Validation Instrument for Undergraduate Qualitative Research Cap Requirements For Instrument Validation download the all common checklist for cap accreditation programs, which contains all the requirements and instructions for. learn how to comply with cap requirements for analytical validation or verification of nonwaived tests, methods, or instruments. find the cap accreditation program requirements for various subdisciplines of pathology and laboratory. as specified in the cap lap chemistry and. Cap Requirements For Instrument Validation.
From www.researchgate.net
Validation and reliability of instruments used in the study. Download Cap Requirements For Instrument Validation with this guideline update, validation requirements for all predictive markers have been harmonized. learn how to meet clia and lap requirements for calibration verification and linearity testing of analytical. as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. find the cap accreditation program requirements for. Cap Requirements For Instrument Validation.
From dxoitzmoz.blob.core.windows.net
Instrument Description And Validation at Carol Randle blog Cap Requirements For Instrument Validation learn how to verify the accuracy and range of your laboratory instruments with the cap cvl program. as specified in the cap lap chemistry and toxicology checklist, laboratories are required to verify the amr at least every. 2 college of american pathologists &doleudwlrq 9hul¿fdwlrq /lqhdulw\ 6xuyh\v how to make the most of your. learn how to. Cap Requirements For Instrument Validation.