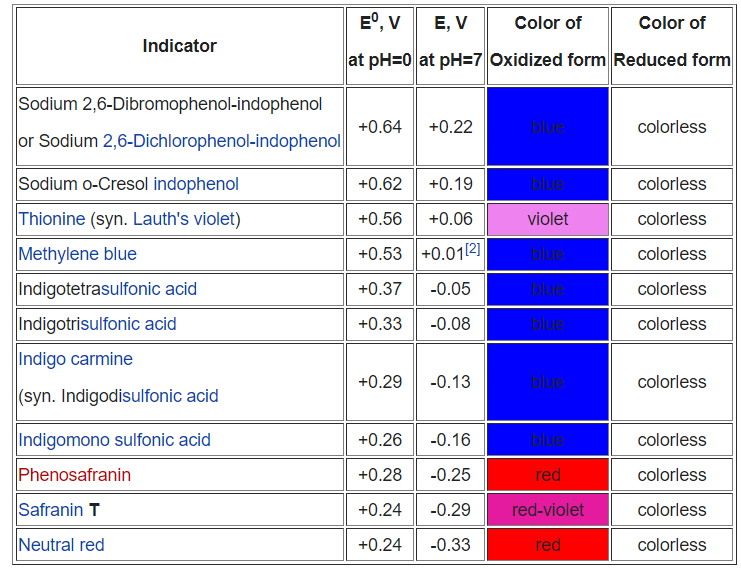
Internal Indicators Used In Titration . precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. When one of the reactants is. internal indicators are generally used in redox titrations. An example of an internal indicator is potassium. when a chemical species is externally added to act as an indicator is called an external indicator. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. the end point of the titration as indicated earlier has to be defined with the help of an indicator. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values.
from school.careers360.com
redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. An example of an internal indicator is potassium. when a chemical species is externally added to act as an indicator is called an external indicator. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. the end point of the titration as indicated earlier has to be defined with the help of an indicator. When one of the reactants is. internal indicators are generally used in redox titrations. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique.
redox titration Overview, Structure, Properties & Uses
Internal Indicators Used In Titration redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. when a chemical species is externally added to act as an indicator is called an external indicator. When one of the reactants is. An example of an internal indicator is potassium. internal indicators are generally used in redox titrations. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. the end point of the titration as indicated earlier has to be defined with the help of an indicator.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. An example of an internal indicator is potassium. the end point of. Internal Indicators Used In Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Internal Indicators Used In Titration precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. internal indicators are generally used in redox titrations. When one of the reactants is. the end point of the titration as indicated earlier has to be defined with the help of an indicator. titration is a typical laboratory method. Internal Indicators Used In Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Internal Indicators Used In Titration the end point of the titration as indicated earlier has to be defined with the help of an indicator. When one of the reactants is. internal indicators are generally used in redox titrations. An example of an internal indicator is potassium. precipitation titration is a type of titration which involves the formation of precipitate during the titration. Internal Indicators Used In Titration.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Internal Indicators Used In Titration internal indicators are generally used in redox titrations. When one of the reactants is. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. the end point of the titration as indicated earlier has to be defined with the help of an indicator. when a chemical species is externally. Internal Indicators Used In Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Internal Indicators Used In Titration An example of an internal indicator is potassium. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. internal indicators are generally used in redox titrations. the end point of the titration as indicated earlier has to be defined with the help of. Internal Indicators Used In Titration.
From www.slideserve.com
PPT Acid Base Review PowerPoint Presentation, free download ID2814652 Internal Indicators Used In Titration When one of the reactants is. internal indicators are generally used in redox titrations. when a chemical species is externally added to act as an indicator is called an external indicator. the end point of the titration as indicated earlier has to be defined with the help of an indicator. An example of an internal indicator is. Internal Indicators Used In Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Internal Indicators Used In Titration the end point of the titration as indicated earlier has to be defined with the help of an indicator. when a chemical species is externally added to act as an indicator is called an external indicator. internal indicators are generally used in redox titrations. An example of an internal indicator is potassium. redox indicator is an. Internal Indicators Used In Titration.
From www.slideserve.com
PPT Volumetric ANALYSIS/TITRATION PowerPoint Presentation, free Internal Indicators Used In Titration internal indicators are generally used in redox titrations. the end point of the titration as indicated earlier has to be defined with the help of an indicator. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. When one of the reactants is. when a. Internal Indicators Used In Titration.
From www.chegg.com
Solved Titration Indicators 8 5. Identify two reasons why Internal Indicators Used In Titration precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. the end point of the titration as indicated earlier has to be defined with the help. Internal Indicators Used In Titration.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Internal Indicators Used In Titration redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. when a chemical species is externally added to act as an indicator is called an external indicator. precipitation titration is a type of titration which involves the formation of precipitate during the titration. Internal Indicators Used In Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. When one of the reactants is. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. internal indicators are generally used in redox titrations. the end point of. Internal Indicators Used In Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Internal Indicators Used In Titration When one of the reactants is. the end point of the titration as indicated earlier has to be defined with the help of an indicator. An example of an internal indicator is potassium. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. . Internal Indicators Used In Titration.
From www.youtube.com
Redox titration using Internal indicator YouTube Internal Indicators Used In Titration when a chemical species is externally added to act as an indicator is called an external indicator. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. internal indicators are generally used in redox titrations. the end point of the titration as indicated earlier has. Internal Indicators Used In Titration.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. An example of an internal indicator is potassium. when a chemical species. Internal Indicators Used In Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. internal indicators are generally used in redox titrations. An example of an internal indicator is potassium. When one of the reactants is. precipitation titration is a type of titration which involves the formation of precipitate during. Internal Indicators Used In Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Internal Indicators Used In Titration redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. An example of an internal indicator is potassium. When one of the reactants is. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. titration is. Internal Indicators Used In Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. when a chemical species is externally added to act as an indicator is called an external indicator. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states,. Internal Indicators Used In Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Internal Indicators Used In Titration precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. when a chemical species is externally added to act as an indicator is called an external indicator. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. internal. Internal Indicators Used In Titration.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Internal Indicators Used In Titration precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. When one of the reactants is. the end point of the titration as indicated earlier has. Internal Indicators Used In Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. when a chemical species is externally added to act as an indicator is called an external indicator. An example. Internal Indicators Used In Titration.
From collegedunia.com
Titration Formula Types, Process & Acid Base Indicators Internal Indicators Used In Titration When one of the reactants is. internal indicators are generally used in redox titrations. when a chemical species is externally added to act as an indicator is called an external indicator. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. redox indicator is an. Internal Indicators Used In Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. internal indicators are generally used in redox titrations. When one of the reactants is. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. the end point of. Internal Indicators Used In Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Internal Indicators Used In Titration when a chemical species is externally added to act as an indicator is called an external indicator. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. When one of the reactants is. An example of an internal indicator is potassium. titration is. Internal Indicators Used In Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Internal Indicators Used In Titration precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. An example of an internal indicator is potassium. titration is a typical laboratory method of quantitative. Internal Indicators Used In Titration.
From mungfali.com
Acid Base Titration Indicator Internal Indicators Used In Titration the end point of the titration as indicated earlier has to be defined with the help of an indicator. when a chemical species is externally added to act as an indicator is called an external indicator. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte.. Internal Indicators Used In Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Internal Indicators Used In Titration titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. When one of the reactants is. when a chemical species is externally added to act as an indicator is. Internal Indicators Used In Titration.
From dxobsfqym.blob.core.windows.net
Titration Indicators Meaning at Angela Armstrong blog Internal Indicators Used In Titration When one of the reactants is. An example of an internal indicator is potassium. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. internal indicators. Internal Indicators Used In Titration.
From jeparatd.blogspot.com
Why Does The Indicator Change Color In Titration Jeparat Internal Indicators Used In Titration precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. When one of the reactants is. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. redox indicator is an organic compound that exhibit a change in color in. Internal Indicators Used In Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Internal Indicators Used In Titration When one of the reactants is. the end point of the titration as indicated earlier has to be defined with the help of an indicator. An example of an internal indicator is potassium. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. internal indicators are generally used in redox. Internal Indicators Used In Titration.
From www.slideshare.net
Acid base titration Internal Indicators Used In Titration when a chemical species is externally added to act as an indicator is called an external indicator. redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. precipitation titration is a type of titration which involves the formation of precipitate during the titration. Internal Indicators Used In Titration.
From studylib.net
AcidBase Titrations Indicators Analytical Chemistry Internal Indicators Used In Titration when a chemical species is externally added to act as an indicator is called an external indicator. internal indicators are generally used in redox titrations. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. titration is a typical laboratory method of quantitative chemical analysis that is used to. Internal Indicators Used In Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Internal Indicators Used In Titration redox indicator is an organic compound that exhibit a change in color in both the reduced and oxidized states, or at various potential values. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of a specified analyte. When one of the reactants is. internal indicators are generally used in. Internal Indicators Used In Titration.
From www.studypool.com
SOLUTION Acid base indicators titration curves Studypool Internal Indicators Used In Titration when a chemical species is externally added to act as an indicator is called an external indicator. When one of the reactants is. internal indicators are generally used in redox titrations. An example of an internal indicator is potassium. titration is a typical laboratory method of quantitative chemical analysis that is used to quantify the concentration of. Internal Indicators Used In Titration.
From school.careers360.com
redox titration Overview, Structure, Properties & Uses Internal Indicators Used In Titration the end point of the titration as indicated earlier has to be defined with the help of an indicator. When one of the reactants is. An example of an internal indicator is potassium. when a chemical species is externally added to act as an indicator is called an external indicator. precipitation titration is a type of titration. Internal Indicators Used In Titration.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Internal Indicators Used In Titration internal indicators are generally used in redox titrations. the end point of the titration as indicated earlier has to be defined with the help of an indicator. precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. When one of the reactants is. redox indicator is an organic compound. Internal Indicators Used In Titration.