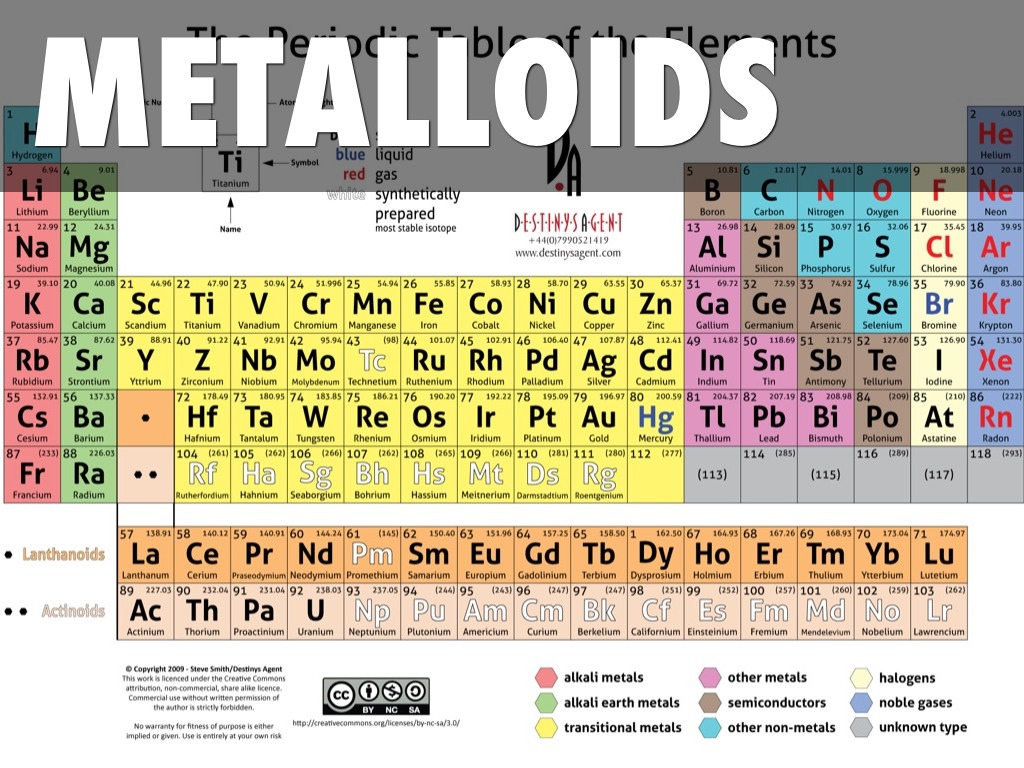
Metalloids Are Generally Hard Lustrous And Malleable . Thus metals are electropositive elements. There are 3 types of elements: All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Properties of metalloids or semimetals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Metals are generally shiny, malleable, and good conductors of heat and electricity. They can form alloys with other metals. They often have high melting and boiling points due to strong interatomic forces. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Lithium, potassium and sodium) corrode in air or seawater; Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids or semimetals possess some properties of metals and some of nonmetals. On the other hand, metals are generally solid (with the. Metalloids exhibit metallic luster and may look like metals. Metalloids are brittle solids that crumble to powder when struck.
from www.haikudeck.com
Thus metals are electropositive elements. They often have high melting and boiling points due to strong interatomic forces. Lithium, potassium and sodium) corrode in air or seawater; Properties of metalloids or semimetals. Metalloids are brittle solids that crumble to powder when struck. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. There are 3 types of elements: On the other hand, metals are generally solid (with the. They can form alloys with other metals. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals.
Metalloids by Megan Maul
Metalloids Are Generally Hard Lustrous And Malleable Lithium, potassium and sodium) corrode in air or seawater; They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. On the other hand, metals are generally solid (with the. Metalloids or semimetals possess some properties of metals and some of nonmetals. Lithium, potassium and sodium) corrode in air or seawater; Properties of metalloids or semimetals. There are 3 types of elements: Metals are generally shiny, malleable, and good conductors of heat and electricity. Metalloids are brittle solids that crumble to powder when struck. They can form alloys with other metals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids exhibit metallic luster and may look like metals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Thus metals are electropositive elements. They often have high melting and boiling points due to strong interatomic forces.
From www.chemistrylearner.com
Metalloids Chemistry Learner Metalloids Are Generally Hard Lustrous And Malleable Thus metals are electropositive elements. Properties of metalloids or semimetals. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids are brittle solids that crumble to powder when struck. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Metalloids or semimetals possess some properties of metals and some of nonmetals.. Metalloids Are Generally Hard Lustrous And Malleable.
From pediabay.com
Metalloids of the Periodic Table Pediabay Metalloids Are Generally Hard Lustrous And Malleable There are 3 types of elements: They often have high melting and boiling points due to strong interatomic forces. Metalloids exhibit metallic luster and may look like metals. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metalloids are brittle solids that crumble to powder when struck. Examples of metalloids. Metalloids Are Generally Hard Lustrous And Malleable.
From knordslearning.com
Periodic Table Metals, Nonmetals & Metalloids (With Images) Metalloids Are Generally Hard Lustrous And Malleable All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Metals are generally shiny, malleable, and good conductors of heat and electricity. There are 3 types of elements: Properties of metalloids or semimetals. They often have high melting and boiling points due. Metalloids Are Generally Hard Lustrous And Malleable.
From edutechspot.com
Metalloids are located where on the periodic table? Here >>> Metalloids Are Generally Hard Lustrous And Malleable On the other hand, metals are generally solid (with the. Lithium, potassium and sodium) corrode in air or seawater; Metalloids are brittle solids that crumble to powder when struck. They can form alloys with other metals. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metalloids exhibit metallic luster and. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
2) Understanding Matter ppt download Metalloids Are Generally Hard Lustrous And Malleable They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metals are generally shiny, malleable, and good conductors of heat and electricity. On the other hand, metals are generally solid (with the. Metalloids are brittle solids that crumble to powder when struck. They often have high melting and boiling points due. Metalloids Are Generally Hard Lustrous And Malleable.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Metalloids Are Generally Hard Lustrous And Malleable They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metals are generally shiny, malleable, and good conductors of heat and electricity. On the other hand, metals are generally solid (with the. Properties of metalloids or semimetals. There are 3 types of elements: They can form alloys with other metals. Metalloids. Metalloids Are Generally Hard Lustrous And Malleable.
From www.adda247.com
What are Metalloids? Definition, Properties and Example Metalloids Are Generally Hard Lustrous And Malleable Thus metals are electropositive elements. Metalloids are brittle solids that crumble to powder when struck. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids exhibit metallic luster and may look like metals. They often have high melting and boiling points due to strong interatomic forces. They are characterized by bright luster,. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
METAL NON METAL Alkali Alkaline Earth Metals Transition Metalloids Metalloids Are Generally Hard Lustrous And Malleable They often have high melting and boiling points due to strong interatomic forces. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Lithium, potassium and sodium) corrode in air or seawater; Metalloids or semimetals possess some properties of metals and some of nonmetals. All elements except hydrogen, which. Metalloids Are Generally Hard Lustrous And Malleable.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Metalloids Are Generally Hard Lustrous And Malleable On the other hand, metals are generally solid (with the. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. They often have high melting and boiling points due to strong interatomic forces. Examples. Metalloids Are Generally Hard Lustrous And Malleable.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Metalloids Are Generally Hard Lustrous And Malleable Metalloids or semimetals possess some properties of metals and some of nonmetals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Metals are generally shiny, malleable, and good conductors of heat and electricity. There are 3 types of elements: Metalloids exhibit metallic luster and may look like metals. Metalloids are brittle solids that crumble to powder when struck.. Metalloids Are Generally Hard Lustrous And Malleable.
From www.youtube.com
Metals, Nonmetals, and Metalloids on the Periodic Table YouTube Metalloids Are Generally Hard Lustrous And Malleable Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metals are generally shiny, malleable, and good conductors of heat and electricity. There are 3 types of elements: Thus metals are electropositive elements. Lithium, potassium and sodium) corrode in air or seawater; They can form alloys with other metals.. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
Science and technology ppt download Metalloids Are Generally Hard Lustrous And Malleable Lithium, potassium and sodium) corrode in air or seawater; Metalloids exhibit metallic luster and may look like metals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. On the other hand, metals are generally solid (with the. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors.. Metalloids Are Generally Hard Lustrous And Malleable.
From exozzfrsb.blob.core.windows.net
Metalloids Are Two Elements at Scott Hickson blog Metalloids Are Generally Hard Lustrous And Malleable Lithium, potassium and sodium) corrode in air or seawater; They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metalloids are brittle solids that crumble to powder when struck. On the other hand, metals are generally solid (with the.. Metalloids Are Generally Hard Lustrous And Malleable.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Metalloids Are Generally Hard Lustrous And Malleable They can form alloys with other metals. There are 3 types of elements: Metalloids or semimetals possess some properties of metals and some of nonmetals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Metalloids exhibit metallic luster and may look like metals. Properties of metalloids or semimetals. Thus metals are electropositive elements. Metals are generally shiny, malleable,. Metalloids Are Generally Hard Lustrous And Malleable.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Metalloids Are Generally Hard Lustrous And Malleable Properties of metalloids or semimetals. There are 3 types of elements: Metalloids are brittle solids that crumble to powder when struck. On the other hand, metals are generally solid (with the. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metalloids or semimetals possess some properties of metals and some. Metalloids Are Generally Hard Lustrous And Malleable.
From homedeso.vercel.app
Metals And Metalloids On Periodic Table Metalloids Are Generally Hard Lustrous And Malleable Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. They can form alloys with other metals. Metalloids are brittle solids that crumble to powder when struck. Metalloids exhibit metallic luster and may look like metals. Thus metals. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Metalloids Are Generally Hard Lustrous And Malleable Properties of metalloids or semimetals. They can form alloys with other metals. Metals are generally shiny, malleable, and good conductors of heat and electricity. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids or semimetals possess some properties of metals and some of nonmetals. Thus metals are electropositive elements. There are. Metalloids Are Generally Hard Lustrous And Malleable.
From knordslearning.com
Metalloids Periodic Table (With Images) Metalloids Are Generally Hard Lustrous And Malleable Metalloids are brittle solids that crumble to powder when struck. They often have high melting and boiling points due to strong interatomic forces. They can form alloys with other metals. There are 3 types of elements: Lithium, potassium and sodium) corrode in air or seawater; Metalloids or semimetals possess some properties of metals and some of nonmetals. Properties of metalloids. Metalloids Are Generally Hard Lustrous And Malleable.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Metalloids Are Generally Hard Lustrous And Malleable There are 3 types of elements: They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. They can form alloys with other metals. Properties of metalloids or semimetals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. All elements except hydrogen, which form positive ions by losing electrons during. Metalloids Are Generally Hard Lustrous And Malleable.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Metalloids Are Generally Hard Lustrous And Malleable Metalloids are brittle solids that crumble to powder when struck. They often have high melting and boiling points due to strong interatomic forces. There are 3 types of elements: Metals are generally shiny, malleable, and good conductors of heat and electricity. They can form alloys with other metals. Thus metals are electropositive elements. Metalloids or semimetals possess some properties of. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
Unit 4 Periodic Table. ppt download Metalloids Are Generally Hard Lustrous And Malleable They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metals are generally shiny, malleable, and good conductors of heat and electricity. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metalloids exhibit metallic luster and may look like metals. Lithium, potassium and sodium) corrode in air or. Metalloids Are Generally Hard Lustrous And Malleable.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Metalloids Are Generally Hard Lustrous And Malleable Metals are generally shiny, malleable, and good conductors of heat and electricity. There are 3 types of elements: Lithium, potassium and sodium) corrode in air or seawater; On the other hand, metals are generally solid (with the. They often have high melting and boiling points due to strong interatomic forces. Metalloids are brittle solids that crumble to powder when struck.. Metalloids Are Generally Hard Lustrous And Malleable.
From sciencetrends.com
4 Properties Of Metalloids Science Trends Metalloids Are Generally Hard Lustrous And Malleable Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Metals are generally shiny, malleable, and good conductors of heat and electricity. They often have high melting and boiling points due to strong interatomic forces. Metalloids are brittle solids that crumble to powder when struck. Metalloids or semimetals possess some properties of metals and some of nonmetals. Properties of. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
Chapter 6 The Periodic Table ppt download Metalloids Are Generally Hard Lustrous And Malleable There are 3 types of elements: Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metals are generally shiny, malleable, and good conductors of heat and electricity. Lithium, potassium and sodium). Metalloids Are Generally Hard Lustrous And Malleable.
From www.slideshare.net
Metals, nonmetals, metalloids Metalloids Are Generally Hard Lustrous And Malleable There are 3 types of elements: Properties of metalloids or semimetals. They often have high melting and boiling points due to strong interatomic forces. They can form alloys with other metals. Metalloids or semimetals possess some properties of metals and some of nonmetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus. Metalloids Are Generally Hard Lustrous And Malleable.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1462875 Metalloids Are Generally Hard Lustrous And Malleable Thus metals are electropositive elements. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metals are generally shiny, malleable, and good conductors of heat and electricity. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. They can form alloys with other metals. They often have high melting and boiling points due to strong interatomic forces.. Metalloids Are Generally Hard Lustrous And Malleable.
From www.slideserve.com
PPT Metals, Nonmetals, and Metalloids PowerPoint Presentation, free Metalloids Are Generally Hard Lustrous And Malleable Metals are generally shiny, malleable, and good conductors of heat and electricity. Metalloids are brittle solids that crumble to powder when struck. On the other hand, metals are generally solid (with the. Metalloids or semimetals possess some properties of metals and some of nonmetals. Lithium, potassium and sodium) corrode in air or seawater; There are 3 types of elements: They. Metalloids Are Generally Hard Lustrous And Malleable.
From www.slideserve.com
PPT Chapter 3 Elements, Compounds, and the Periodic Table PowerPoint Metalloids Are Generally Hard Lustrous And Malleable Metalloids are brittle solids that crumble to powder when struck. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Lithium, potassium and sodium) corrode in air or seawater; They often have high melting and boiling points due to strong interatomic forces. Metalloids or semimetals possess some properties of metals and. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
PERIODIC TABLE. ppt download Metalloids Are Generally Hard Lustrous And Malleable There are 3 types of elements: Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Properties of metalloids or semimetals. On the other hand, metals are generally solid (with the. They. Metalloids Are Generally Hard Lustrous And Malleable.
From www.haikudeck.com
Metalloids by Megan Maul Metalloids Are Generally Hard Lustrous And Malleable They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. Thus metals are electropositive elements. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. They can form alloys. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
PERIODIC TABLE. ppt download Metalloids Are Generally Hard Lustrous And Malleable They often have high melting and boiling points due to strong interatomic forces. There are 3 types of elements: All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Lithium, potassium and sodium) corrode in air or seawater; On the other hand, metals are generally solid (with the. Examples of metalloids include boron,. Metalloids Are Generally Hard Lustrous And Malleable.
From blog.thepipingmart.com
Metalloids Uses and Properties Metalloids Are Generally Hard Lustrous And Malleable Properties of metalloids or semimetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. There are 3 types of elements: Metalloids or semimetals possess some properties of metals and some of nonmetals. They often have high melting and boiling points due to strong interatomic forces. Metals are generally. Metalloids Are Generally Hard Lustrous And Malleable.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table Metalloids Are Generally Hard Lustrous And Malleable They often have high melting and boiling points due to strong interatomic forces. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Lithium, potassium and sodium) corrode in air or seawater; Properties of metalloids or semimetals. Thus metals are electropositive elements. Metalloids are brittle solids that crumble to powder when struck. Metals. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
Chapter 6 “The Periodic Table” ppt video online download Metalloids Are Generally Hard Lustrous And Malleable Metalloids are brittle solids that crumble to powder when struck. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Examples of metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. They can form alloys with other metals. There are 3 types of elements: Thus metals are electropositive elements. Metalloids exhibit metallic luster. Metalloids Are Generally Hard Lustrous And Malleable.
From slideplayer.com
Periodic Table and Chemical Properties ppt download Metalloids Are Generally Hard Lustrous And Malleable Metalloids are brittle solids that crumble to powder when struck. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Properties of metalloids or semimetals. Metals are generally shiny, malleable, and good conductors of heat and electricity. They can form alloys with other metals. They are characterized by bright. Metalloids Are Generally Hard Lustrous And Malleable.