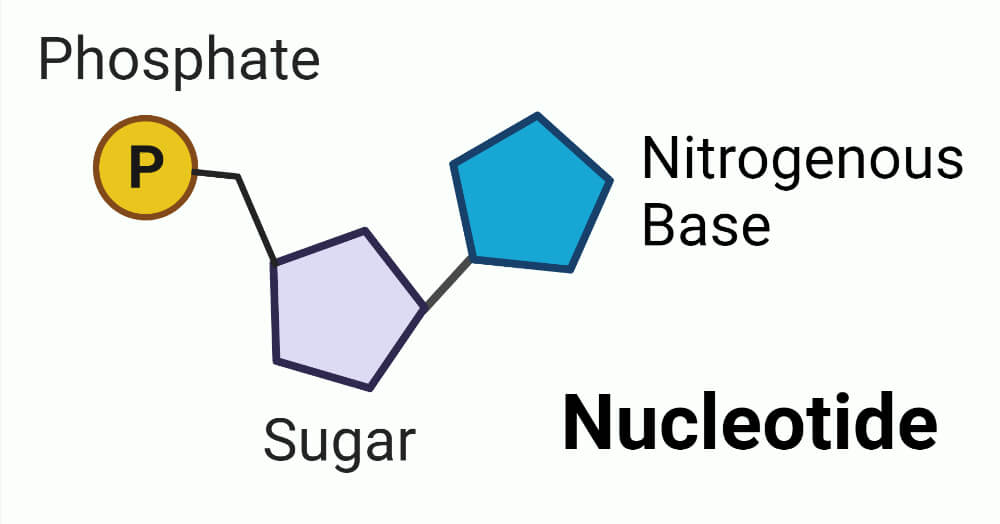
Bases Definition In Biology . bases are molecules that can split apart in water and release hydroxide ions. some are bases and some are neither acids nor bases. Like strong acids, strong bases can harm organisms and damage materials. A solution with a ph higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. The unshared pair of electrons serves as a. The most common example is sodium hydroxide (naoh). but the most common bases are those molecules that contain an amino group (7). To understand acids and bases, you need to know more about pure water. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. In pure water (such as. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a.
from microbenotes.com
but the most common bases are those molecules that contain an amino group (7). bases are molecules that can split apart in water and release hydroxide ions. The most common example is sodium hydroxide (naoh). If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. Bases, such as baking soda, have a bitter taste. The unshared pair of electrons serves as a. To understand acids and bases, you need to know more about pure water. In pure water (such as. some are bases and some are neither acids nor bases. A solution with a ph higher than 7 is called a base.
Nucleotide Definition, Characteristics, Biosynthesis, Functions
Bases Definition In Biology Like strong acids, strong bases can harm organisms and damage materials. but the most common bases are those molecules that contain an amino group (7). Bases, such as baking soda, have a bitter taste. The unshared pair of electrons serves as a. The most common example is sodium hydroxide (naoh). (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. some are bases and some are neither acids nor bases. A solution with a ph higher than 7 is called a base. In pure water (such as. To understand acids and bases, you need to know more about pure water. bases are molecules that can split apart in water and release hydroxide ions. Like strong acids, strong bases can harm organisms and damage materials.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable Bases Definition In Biology bases are molecules that can split apart in water and release hydroxide ions. A solution with a ph higher than 7 is called a base. To understand acids and bases, you need to know more about pure water. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. but the most common bases. Bases Definition In Biology.
From www.slideshare.net
Biology base and acid Bases Definition In Biology Like strong acids, strong bases can harm organisms and damage materials. bases are molecules that can split apart in water and release hydroxide ions. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. The most common example is sodium hydroxide (naoh). To understand acids and bases, you need to know more about pure. Bases Definition In Biology.
From www.worksheetsplanet.com
What is a Base Definition of Base Bases Definition In Biology In pure water (such as. To understand acids and bases, you need to know more about pure water. Like strong acids, strong bases can harm organisms and damage materials. but the most common bases are those molecules that contain an amino group (7). (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. If. Bases Definition In Biology.
From www.slideserve.com
PPT DNA and Genes PowerPoint Presentation ID6178768 Bases Definition In Biology but the most common bases are those molecules that contain an amino group (7). Like strong acids, strong bases can harm organisms and damage materials. The unshared pair of electrons serves as a. To understand acids and bases, you need to know more about pure water. Bases, such as baking soda, have a bitter taste. If a solution has. Bases Definition In Biology.
From www.slideserve.com
PPT Genes and DNA PowerPoint Presentation, free download ID1819104 Bases Definition In Biology (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. A solution with a ph higher than 7 is called a base. some are bases and some are neither acids nor bases. bases are molecules that can split apart in water and release hydroxide ions. In pure water (such as. To understand acids. Bases Definition In Biology.
From malaysianstudentstudynotes.blogspot.com
Student Study Notes STPM Biology Biological Molecules Part 17 Nucleic Bases Definition In Biology but the most common bases are those molecules that contain an amino group (7). some are bases and some are neither acids nor bases. A solution with a ph higher than 7 is called a base. To understand acids and bases, you need to know more about pure water. (1) (molecular biology) the nucleobase of a nucleotide involved. Bases Definition In Biology.
From www.biologyonline.com
Nucleotide Definition and Examples Biology Online Dictionary Bases Definition In Biology but the most common bases are those molecules that contain an amino group (7). A solution with a ph higher than 7 is called a base. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. bases are molecules that can split apart in water and release hydroxide. Bases Definition In Biology.
From biologydictionary.net
Base Pair Definition, Rules and Quiz Biology Dictionary Bases Definition In Biology The most common example is sodium hydroxide (naoh). (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. but the most common bases are those molecules that contain an amino group (7). bases are molecules that can split apart in water and release hydroxide ions. In pure water (such as. To understand acids. Bases Definition In Biology.
From worksheetmediagaertner.z13.web.core.windows.net
Base Pairing Of Dna Bases Definition In Biology In pure water (such as. To understand acids and bases, you need to know more about pure water. The unshared pair of electrons serves as a. A solution with a ph higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. If a solution has a lower concentration of hydronium ions than pure water,. Bases Definition In Biology.
From www.slideserve.com
PPT DNA and Genes PowerPoint Presentation, free download ID5759540 Bases Definition In Biology (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. but the most common bases are those molecules that contain an amino group (7). A solution with a ph higher than 7 is called a base. bases are molecules that can split apart in water and release hydroxide ions. In pure water (such. Bases Definition In Biology.
From www.genome.gov
Base Pair Bases Definition In Biology (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. Like strong acids, strong bases can harm organisms and damage materials. In pure water (such as. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. but the most common bases are those molecules. Bases Definition In Biology.
From drawittoknowit.com
Cell Biology Glossary DNA Base Pairing Draw It to Know It Bases Definition In Biology In pure water (such as. but the most common bases are those molecules that contain an amino group (7). (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. The unshared pair of electrons serves as a. To understand acids and bases, you need to know more about pure water. If a solution has. Bases Definition In Biology.
From www.slideserve.com
PPT Acids & Bases What’s the difference??? PowerPoint Presentation Bases Definition In Biology but the most common bases are those molecules that contain an amino group (7). The unshared pair of electrons serves as a. Like strong acids, strong bases can harm organisms and damage materials. Bases, such as baking soda, have a bitter taste. To understand acids and bases, you need to know more about pure water. (1) (molecular biology) the. Bases Definition In Biology.
From www.biologyonline.com
Basepairing rule Definition and Examples Biology Online Dictionary Bases Definition In Biology (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. some are bases and some are neither acids nor bases. bases are molecules that can split apart in water and release hydroxide ions. To understand acids and bases, you need to know more about pure water. If a solution has a lower concentration. Bases Definition In Biology.
From quizdbcornwallis.z21.web.core.windows.net
What Is A Base Biology Definition Bases Definition In Biology A solution with a ph higher than 7 is called a base. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. some are bases and some are neither acids nor bases. bases are molecules that can split apart in water and release hydroxide ions. To understand acids. Bases Definition In Biology.
From www.slideshare.net
Biology base and acid Bases Definition In Biology bases are molecules that can split apart in water and release hydroxide ions. Bases, such as baking soda, have a bitter taste. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. In pure water (such as. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing,. Bases Definition In Biology.
From www.slideserve.com
PPT DNA PowerPoint Presentation, free download ID2648369 Bases Definition In Biology The most common example is sodium hydroxide (naoh). but the most common bases are those molecules that contain an amino group (7). To understand acids and bases, you need to know more about pure water. Bases, such as baking soda, have a bitter taste. some are bases and some are neither acids nor bases. Like strong acids, strong. Bases Definition In Biology.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID4339426 Bases Definition In Biology bases are molecules that can split apart in water and release hydroxide ions. Like strong acids, strong bases can harm organisms and damage materials. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. To understand acids and bases, you need to know more about pure water. Bases, such. Bases Definition In Biology.
From byjus.com
Nucleotide Structure, Examples and Function Bases Definition In Biology In pure water (such as. Like strong acids, strong bases can harm organisms and damage materials. To understand acids and bases, you need to know more about pure water. A solution with a ph higher than 7 is called a base. The unshared pair of electrons serves as a. The most common example is sodium hydroxide (naoh). some are. Bases Definition In Biology.
From definitionklw.blogspot.com
Nitrogenous Base Definition Biology DEFINITION KLW Bases Definition In Biology To understand acids and bases, you need to know more about pure water. but the most common bases are those molecules that contain an amino group (7). some are bases and some are neither acids nor bases. The unshared pair of electrons serves as a. bases are molecules that can split apart in water and release hydroxide. Bases Definition In Biology.
From www.pinterest.com
Nitrogenous base definition of nitrogenous base by Medical dictionary Bases Definition In Biology Like strong acids, strong bases can harm organisms and damage materials. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. To understand acids and bases, you need to know more about pure water. bases are molecules that can split apart in water and release hydroxide ions. The unshared. Bases Definition In Biology.
From www.geeksforgeeks.org
What are Bases? Definition, Examples, Types, Properties and Uses Bases Definition In Biology some are bases and some are neither acids nor bases. Bases, such as baking soda, have a bitter taste. but the most common bases are those molecules that contain an amino group (7). The most common example is sodium hydroxide (naoh). A solution with a ph higher than 7 is called a base. If a solution has a. Bases Definition In Biology.
From www.slideserve.com
PPT Organic Molecules PowerPoint Presentation, free download ID2672764 Bases Definition In Biology The unshared pair of electrons serves as a. In pure water (such as. but the most common bases are those molecules that contain an amino group (7). some are bases and some are neither acids nor bases. To understand acids and bases, you need to know more about pure water. Bases, such as baking soda, have a bitter. Bases Definition In Biology.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID1383826 Bases Definition In Biology but the most common bases are those molecules that contain an amino group (7). Bases, such as baking soda, have a bitter taste. The unshared pair of electrons serves as a. A solution with a ph higher than 7 is called a base. Like strong acids, strong bases can harm organisms and damage materials. If a solution has a. Bases Definition In Biology.
From microbenotes.com
Nucleotide Definition, Characteristics, Biosynthesis, Functions Bases Definition In Biology (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. A solution with a ph higher than 7 is called a base. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. Like strong acids, strong bases can harm organisms and damage materials. In pure. Bases Definition In Biology.
From knowgenetics.org
Nucleotides and Bases Generation Bases Definition In Biology Like strong acids, strong bases can harm organisms and damage materials. Bases, such as baking soda, have a bitter taste. some are bases and some are neither acids nor bases. A solution with a ph higher than 7 is called a base. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. In pure. Bases Definition In Biology.
From biology4isc.weebly.com
Nucleic acid BIOLOGY4ISC Bases Definition In Biology but the most common bases are those molecules that contain an amino group (7). The most common example is sodium hydroxide (naoh). some are bases and some are neither acids nor bases. A solution with a ph higher than 7 is called a base. bases are molecules that can split apart in water and release hydroxide ions.. Bases Definition In Biology.
From www.slideshare.net
Biology base and acid Bases Definition In Biology The most common example is sodium hydroxide (naoh). In pure water (such as. some are bases and some are neither acids nor bases. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. but the most common bases are those molecules that contain an amino group (7). If a solution has a lower. Bases Definition In Biology.
From quizdbcornwallis.z21.web.core.windows.net
What Are Bases In Biology Bases Definition In Biology If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. some are bases and some are neither acids nor bases. In pure water (such as. A solution with a ph higher than 7 is. Bases Definition In Biology.
From slidetodoc.com
ACID AND BASE PROPERTIES DEFINITION ACID AND BASES Bases Definition In Biology (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. Like strong acids, strong bases can harm organisms and damage materials. A solution with a ph higher than 7 is called a base. bases are molecules that can split apart in water and release hydroxide ions. The unshared pair of electrons serves as a.. Bases Definition In Biology.
From www.slideserve.com
PPT Definitions of Acids and Bases PowerPoint Presentation, free Bases Definition In Biology (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. but the most common bases are those molecules that contain an amino group (7). If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. A solution with a ph higher than 7 is called. Bases Definition In Biology.
From exolsukgk.blob.core.windows.net
Rna Dna Bases at Randy Molina blog Bases Definition In Biology To understand acids and bases, you need to know more about pure water. bases are molecules that can split apart in water and release hydroxide ions. A solution with a ph higher than 7 is called a base. some are bases and some are neither acids nor bases. In pure water (such as. Bases, such as baking soda,. Bases Definition In Biology.
From www.expii.com
Bases — Definition & Overview Expii Bases Definition In Biology A solution with a ph higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. some are bases and some are neither acids nor bases. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. Like strong acids, strong bases can harm organisms and damage materials. bases. Bases Definition In Biology.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo Bases Definition In Biology The most common example is sodium hydroxide (naoh). Like strong acids, strong bases can harm organisms and damage materials. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. (1) (molecular biology) the nucleobase of a nucleotide involved in base pairing, as of a. The unshared pair of electrons serves. Bases Definition In Biology.
From definitionklw.blogspot.com
Definition Of Base In Science DEFINITION KLW Bases Definition In Biology To understand acids and bases, you need to know more about pure water. If a solution has a lower concentration of hydronium ions than pure water, it has a ph higher than 7. bases are molecules that can split apart in water and release hydroxide ions. Bases, such as baking soda, have a bitter taste. A solution with a. Bases Definition In Biology.