Expanded Octet Worksheet . Recognize the four types of solids: Recognize how the macroscopic properties of. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. (please note, none of the solutions are using the expanded octet rule or formal charges) a. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Network (covalent), metallic, ionic, and molecular. If you are not sure if your structure is correct, do a formal charge check. Draw the lewis structures for each of the following molecules.
from www.chegg.com
In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Recognize the four types of solids: Draw the lewis structures for each of the following molecules. Recognize how the macroscopic properties of. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Network (covalent), metallic, ionic, and molecular. If you are not sure if your structure is correct, do a formal charge check. (please note, none of the solutions are using the expanded octet rule or formal charges) a.
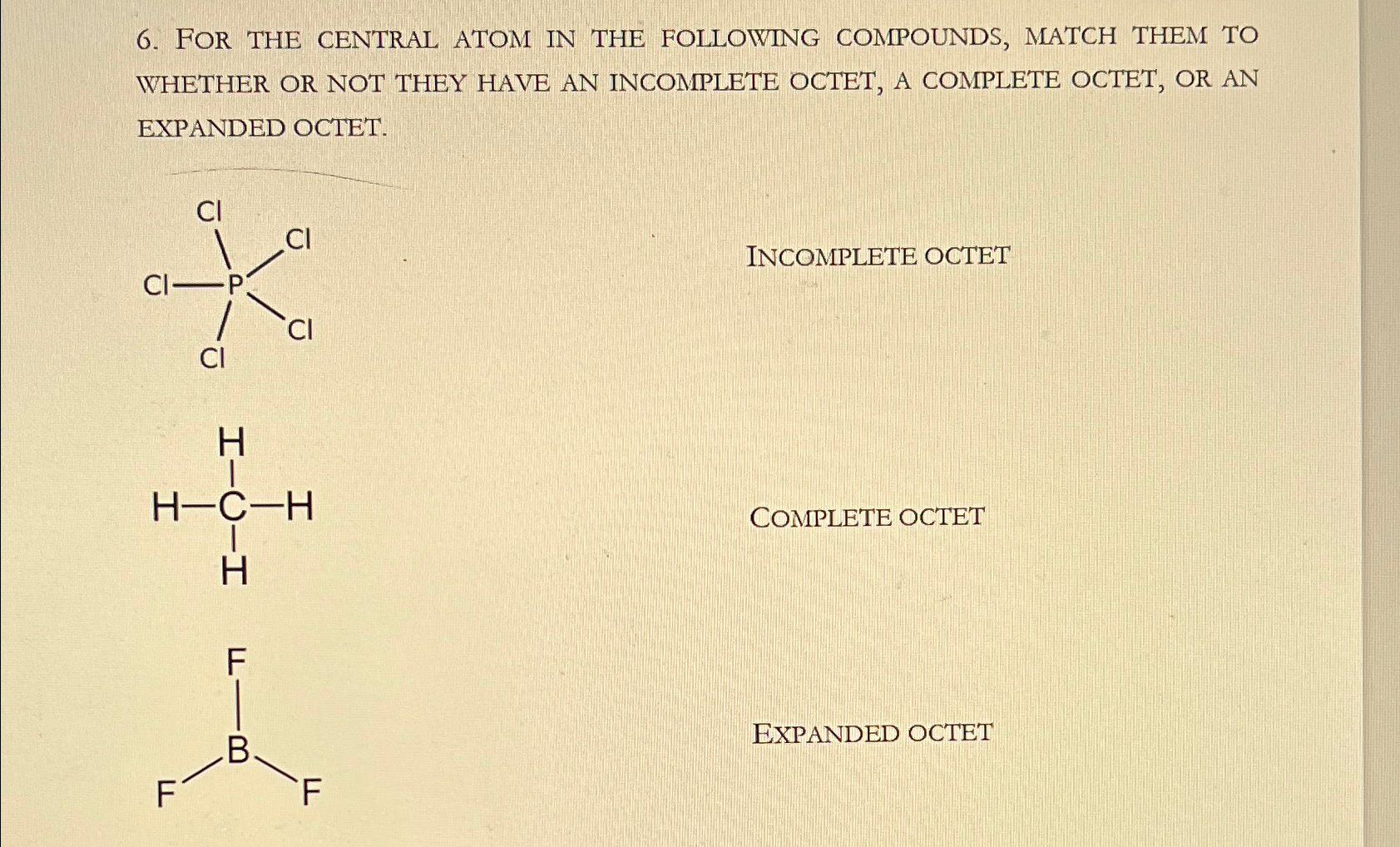
Solved FOR THE CENTRAL ATOM IN THE FOLLOWING COMPOUNDS,
Expanded Octet Worksheet Recognize how the macroscopic properties of. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. (please note, none of the solutions are using the expanded octet rule or formal charges) a. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Network (covalent), metallic, ionic, and molecular. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Recognize how the macroscopic properties of. If you are not sure if your structure is correct, do a formal charge check. Recognize the four types of solids: Draw the lewis structures for each of the following molecules.
From www.chegg.com
Solved (a) State the "Octet Rule" in your own words. (b) Expanded Octet Worksheet If you are not sure if your structure is correct, do a formal charge check. Draw the lewis structures for each of the following molecules. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. (please note, none of the solutions are using the expanded octet rule or formal charges) a. Network. Expanded Octet Worksheet.
From www.slideserve.com
PPT Clicker PowerPoint Presentation, free download ID3918371 Expanded Octet Worksheet Draw the lewis structures for each of the following molecules. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Recognize the four types of solids: Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Recognize how the macroscopic properties of.. Expanded Octet Worksheet.
From www.scribd.com
Expanded Octet Rule Chemsitry PDF Atomic Orbital Covalent Bond Expanded Octet Worksheet Recognize how the macroscopic properties of. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. If you are not sure if your structure is correct, do a. Expanded Octet Worksheet.
From www.youtube.com
Lewis Structures III Expanded octets YouTube Expanded Octet Worksheet Recognize the four types of solids: Draw the lewis structures for each of the following molecules. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Network (covalent), metallic, ionic, and molecular. Recognize how the macroscopic properties of. • to learn the octet rule and how. Expanded Octet Worksheet.
From rumble.com
Lewis diagrams, expanded octet Chemistry Expanded Octet Worksheet Recognize how the macroscopic properties of. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Network (covalent), metallic, ionic, and molecular. (please note, none of the solutions. Expanded Octet Worksheet.
From www.studocu.com
Expanded Octets with Cl CHEM12A Studocu Expanded Octet Worksheet Recognize the four types of solids: Recognize how the macroscopic properties of. If you are not sure if your structure is correct, do a formal charge check. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. (please note, none of the solutions are using the expanded octet rule or. Expanded Octet Worksheet.
From www.animalia-life.club
Expanded Octet Expanded Octet Worksheet • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has. Expanded Octet Worksheet.
From www.youtube.com
Expanded Octets YouTube Expanded Octet Worksheet • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has. Expanded Octet Worksheet.
From es.scribd.com
Polyatomic Ions Hydroxide Acetate Expanded Octet Worksheet (please note, none of the solutions are using the expanded octet rule or formal charges) a. Network (covalent), metallic, ionic, and molecular. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. If you are not sure if your structure is correct, do a formal charge check. Recognize the four types of. Expanded Octet Worksheet.
From www.youtube.com
Expanded Octet Lewis Structures YouTube Expanded Octet Worksheet Draw the lewis structures for each of the following molecules. Network (covalent), metallic, ionic, and molecular. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. If you are not sure if your structure is correct, do a formal charge check. Recognize how the macroscopic properties of. (please note, none. Expanded Octet Worksheet.
From learnwithdrscott.com
Octet Rule Easy Hard Science Expanded Octet Worksheet • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Recognize the four types of solids: If you are not sure if your structure is correct, do a formal charge check. Draw the lewis structures for each of the following molecules. Network (covalent), metallic, ionic, and molecular. In this picture. Expanded Octet Worksheet.
From www.numerade.com
SOLVED BCl3 is an example of (1) octet molecule (2) Expanded octet molecule (3) Odd Expanded Octet Worksheet If you are not sure if your structure is correct, do a formal charge check. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Draw the lewis. Expanded Octet Worksheet.
From www.youtube.com
Lec16 Lewis Structures and Expanded Octets YouTube Expanded Octet Worksheet • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has. Expanded Octet Worksheet.
From www.youtube.com
Expanded Octets & VSEPR YouTube Expanded Octet Worksheet Draw the lewis structures for each of the following molecules. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Recognize the four types of solids: In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. (please note,. Expanded Octet Worksheet.
From www.studypool.com
SOLUTION Electronic Configuration / Octet Rule worksheet SOLVED Studypool Expanded Octet Worksheet • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Network (covalent), metallic, ionic, and molecular. If you are not sure if your structure is correct,. Expanded Octet Worksheet.
From www.chegg.com
Solved Octet , OddElectron Molecules, Expanded Expanded Octet Worksheet If you are not sure if your structure is correct, do a formal charge check. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Network. Expanded Octet Worksheet.
From www.chegg.com
Solved Worksheet 4D Expanded Octet 1. Consider the Expanded Octet Worksheet Recognize the four types of solids: (please note, none of the solutions are using the expanded octet rule or formal charges) a. If you are not sure if your structure is correct, do a formal charge check. Draw the lewis structures for each of the following molecules. Network (covalent), metallic, ionic, and molecular. • to learn the octet rule and. Expanded Octet Worksheet.
From www.youtube.com
Expanded octets YouTube Expanded Octet Worksheet Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Network (covalent), metallic, ionic, and molecular. Recognize the four types of solids: Draw the lewis structures for each of the following molecules. Recognize how the macroscopic properties of. • to learn the octet rule and how to apply it in drawing lewis. Expanded Octet Worksheet.
From ar.inspiredpencil.com
Expanded Octet Chart Expanded Octet Worksheet If you are not sure if your structure is correct, do a formal charge check. Recognize the four types of solids: • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Draw the lewis structures for each of the following molecules. Recognize how the macroscopic properties of. In this picture. Expanded Octet Worksheet.
From www.youtube.com
Lewis structures expanded octets YouTube Expanded Octet Worksheet If you are not sure if your structure is correct, do a formal charge check. Recognize the four types of solids: In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. • to learn the octet rule and how to apply it in drawing lewis structures. Expanded Octet Worksheet.
From www.numerade.com
SOLVEDWhich elements in the list below are capable of forming molecules in which one of its Expanded Octet Worksheet Draw the lewis structures for each of the following molecules. Recognize how the macroscopic properties of. Recognize the four types of solids: Network (covalent), metallic, ionic, and molecular. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. If you are not sure if your structure is correct, do a formal charge. Expanded Octet Worksheet.
From www.chegg.com
Solved Worksheet 4D Expanded Octet 1. Consider the Expanded Octet Worksheet (please note, none of the solutions are using the expanded octet rule or formal charges) a. Draw the lewis structures for each of the following molecules. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. If you are not sure if your structure is correct,. Expanded Octet Worksheet.
From www.chegg.com
Solved Expanded octets Select the correct Lewis structure Expanded Octet Worksheet Network (covalent), metallic, ionic, and molecular. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Draw the lewis structures for each of the following molecules. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. (please note, none of the solutions. Expanded Octet Worksheet.
From www.slideserve.com
PPT 1 10 11 12 15 17 20 24 31 37 39 49 53 55 61 65 67 71 88 103 PowerPoint Presentation ID Expanded Octet Worksheet Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Network (covalent), metallic, ionic, and molecular. Recognize how the macroscopic properties of. If you are not sure if your structure is correct, do a formal charge check. In this picture the central s atom has six f atoms coordinated, but only shares. Expanded Octet Worksheet.
From studylibcurtiss.z13.web.core.windows.net
Octet Rule Worksheet Expanded Octet Worksheet Draw the lewis structures for each of the following molecules. Recognize how the macroscopic properties of. Recognize the four types of solids: (please note, none of the solutions are using the expanded octet rule or formal charges) a. If you are not sure if your structure is correct, do a formal charge check. • to learn the octet rule and. Expanded Octet Worksheet.
From ar.inspiredpencil.com
Expanded Octet Periodic Table Expanded Octet Worksheet Draw the lewis structures for each of the following molecules. Recognize how the macroscopic properties of. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Recognize the. Expanded Octet Worksheet.
From animalia-life.club
Expanded Octet Expanded Octet Worksheet Recognize how the macroscopic properties of. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. (please note, none of the solutions are using the expanded octet rule or formal charges) a. Recognize the four types of solids: Draw the lewis structures for each of the following molecules. Network (covalent),. Expanded Octet Worksheet.
From www.slideserve.com
PPT Lewis Dot Structures PowerPoint Presentation, free download ID6235413 Expanded Octet Worksheet (please note, none of the solutions are using the expanded octet rule or formal charges) a. Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. If you are not sure if your structure is correct, do a formal charge check. Network (covalent), metallic, ionic, and molecular. Recognize the four types of. Expanded Octet Worksheet.
From www.slideserve.com
PPT Intersection 4 PowerPoint Presentation, free download ID6313576 Expanded Octet Worksheet Network (covalent), metallic, ionic, and molecular. Draw the lewis structures for each of the following molecules. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete.. Expanded Octet Worksheet.
From www.chegg.com
Solved Expanded octets Select the correct Lewis structure Expanded Octet Worksheet Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. Network (covalent), metallic, ionic, and molecular. Draw the lewis structures for each of the following molecules. Recognize how the macroscopic properties of. If you are not sure if your structure is correct, do a formal charge check. Recognize the four types of. Expanded Octet Worksheet.
From www.chegg.com
Solved Expanded octets Select the correct Lewis structure Expanded Octet Worksheet Recognize the four types of solids: (please note, none of the solutions are using the expanded octet rule or formal charges) a. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. If you are not sure if your structure is correct, do a formal charge check. In this picture. Expanded Octet Worksheet.
From sciencenotes.org
Octet Rule Definition, Examples, and Exceptions Expanded Octet Worksheet If you are not sure if your structure is correct, do a formal charge check. Network (covalent), metallic, ionic, and molecular. Draw the lewis structures for each of the following molecules. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. (please note, none of the. Expanded Octet Worksheet.
From www.vrogue.co
Illustrated Glossary Of Organic Chemistry Expanded Oc vrogue.co Expanded Octet Worksheet Network (covalent), metallic, ionic, and molecular. Draw the lewis structures for each of the following molecules. Recognize the four types of solids: Recognize how the macroscopic properties of. (please note, none of the solutions are using the expanded octet rule or formal charges) a. If you are not sure if your structure is correct, do a formal charge check. Read. Expanded Octet Worksheet.
From www.animalia-life.club
Expanded Octet Expanded Octet Worksheet In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. • to learn the octet rule and how to apply it in drawing lewis structures for polyatomic ions and molecules. Recognize how the macroscopic properties of. Read the instructions for drawing lewis structures worksheet carefully and. Expanded Octet Worksheet.
From www.chegg.com
Solved FOR THE CENTRAL ATOM IN THE FOLLOWING COMPOUNDS, Expanded Octet Worksheet Recognize the four types of solids: Read the instructions for drawing lewis structures worksheet carefully and complete lewis structures for each of the following. In this picture the central s atom has six f atoms coordinated, but only shares four bonding pairs of electrons and has a complete. (please note, none of the solutions are using the expanded octet rule. Expanded Octet Worksheet.