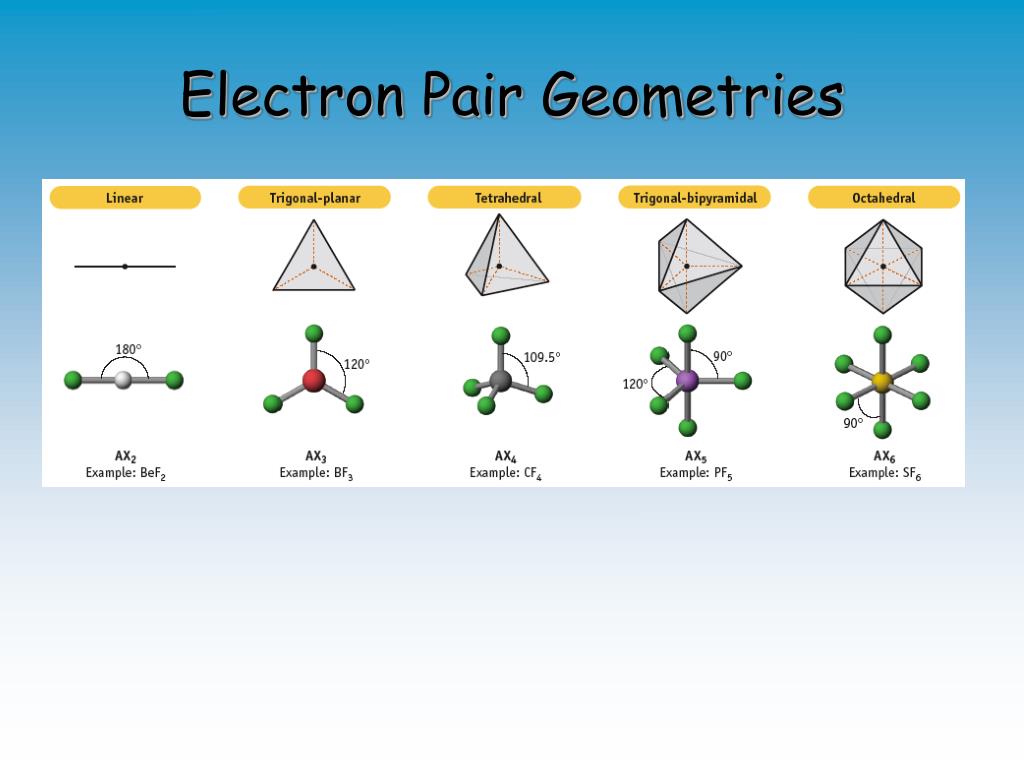
What Is Electronic Geometry . The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. molecular structure describes the location of the atoms, not the electrons. the main difference between electron geometry and molecular geometry is that electron geometry is found by. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. We differentiate between these two situations by naming.
from www.slideserve.com
The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the main difference between electron geometry and molecular geometry is that electron geometry is found by. We differentiate between these two situations by naming. molecular structure describes the location of the atoms, not the electrons. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places.
PPT Bonding and Molecular Structure PowerPoint Presentation, free
What Is Electronic Geometry The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. the main difference between electron geometry and molecular geometry is that electron geometry is found by. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. molecular structure describes the location of the atoms, not the electrons. We differentiate between these two situations by naming. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,.
From numbersaad.weebly.com
Electron geometry chart and examples numbersaad What Is Electronic Geometry We differentiate between these two situations by naming. molecular structure describes the location of the atoms, not the electrons. the main difference between electron geometry and molecular geometry is that electron geometry is found by. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. the premise of the vsepr theory is that electron pairs located. What Is Electronic Geometry.
From techiescientist.com
H3O+ Lewis Structure, Geometry, Hybridization, and MO Diagram What Is Electronic Geometry Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. molecular. What Is Electronic Geometry.
From quizlet.com
Atom's hybridization & electron geometry & Molecular geometry Diagram What Is Electronic Geometry the main difference between electron geometry and molecular geometry is that electron geometry is found by. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt. What Is Electronic Geometry.
From employees.csbsju.edu
molecular geometry What Is Electronic Geometry after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. We differentiate between these two situations by naming. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise. What Is Electronic Geometry.
From www.youtube.com
What is the difference between Electron Domain Geometry & Molecular What Is Electronic Geometry the main difference between electron geometry and molecular geometry is that electron geometry is found by. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the premise of the. What Is Electronic Geometry.
From www.slideserve.com
PPT Bonding and Molecular Structure PowerPoint Presentation, free What Is Electronic Geometry We differentiate between these two situations by naming. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the main difference between electron geometry and molecular geometry is that electron geometry is found by. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. the premise of the. What Is Electronic Geometry.
From www.youtube.com
Electron Geometry for NO3 (Nitrate ion) YouTube What Is Electronic Geometry after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. Bonded atoms and unshared pairs of electrons about a central atom are as. What Is Electronic Geometry.
From courses.lumenlearning.com
Lone Electron Pairs Introduction to Chemistry What Is Electronic Geometry We differentiate between these two situations by naming. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. The vsepr theory detremines molecular. What Is Electronic Geometry.
From www.youtube.com
XeF4 Molecular Geometry, Bond Angles & Electron Geometry YouTube What Is Electronic Geometry the main difference between electron geometry and molecular geometry is that electron geometry is found by. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. after calculating the. What Is Electronic Geometry.
From www.pinterest.com
Electron and Molecular Geometry On Central Atom. Click for Print View What Is Electronic Geometry molecular structure describes the location of the atoms, not the electrons. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. the main difference between electron geometry and molecular geometry is that electron geometry is found by. . What Is Electronic Geometry.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules What Is Electronic Geometry Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. the main difference between electron geometry and molecular geometry is that electron. What Is Electronic Geometry.
From printablemagiclemann.z19.web.core.windows.net
Electron Group Geometry Chart What Is Electronic Geometry molecular structure describes the location of the atoms, not the electrons. the main difference between electron geometry and molecular geometry is that electron geometry is found by. We differentiate between these two situations by naming. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. Bonded atoms and unshared pairs of electrons about a central atom are. What Is Electronic Geometry.
From www.youtube.com
NH2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube What Is Electronic Geometry We differentiate between these two situations by naming. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. molecular structure describes the location of the atoms, not the electrons. . What Is Electronic Geometry.
From www.pinterest.es
Electron Domain Shape Chemistry basics, Chemistry education What Is Electronic Geometry We differentiate between these two situations by naming. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. molecular structure describes the location of the atoms, not the electrons. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the. What Is Electronic Geometry.
From www.youtube.com
Electron Geometry for CO (Carbon monoxide) YouTube What Is Electronic Geometry the main difference between electron geometry and molecular geometry is that electron geometry is found by. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. molecular structure describes the location of the atoms, not the electrons. Bonded atoms and unshared pairs of electrons about a central atom are. What Is Electronic Geometry.
From mavink.com
Vsepr Electron Geometry What Is Electronic Geometry the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. molecular structure describes the location of the atoms, not the electrons. the main difference between electron geometry and molecular geometry is that electron geometry is found by. Bonded atoms and. What Is Electronic Geometry.
From learningcampusdustin.z4.web.core.windows.net
Electron Domain Geometry Chart What Is Electronic Geometry The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the main difference between electron geometry and molecular geometry is. What Is Electronic Geometry.
From fesschange.weebly.com
Electron geometry chart 8 electron groups fesschange What Is Electronic Geometry the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. molecular structure describes the location of the atoms, not the electrons. We differentiate between these two situations by naming. Bonded atoms and unshared pairs of electrons about a central atom are. What Is Electronic Geometry.
From ecbool.weebly.com
Electron domain geometry and molecular geometry chart ecbool What Is Electronic Geometry molecular structure describes the location of the atoms, not the electrons. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. We. What Is Electronic Geometry.
From heryhd.weebly.com
Electron and molecular geometry chart examples heryhd What Is Electronic Geometry The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. We differentiate between these two situations by naming. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. molecular structure describes the location of the atoms, not the electrons. . What Is Electronic Geometry.
From www.pinterest.com
Electron Molecular Geometry Chemistry Molecular geometry, Teaching What Is Electronic Geometry We differentiate between these two situations by naming. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. molecular structure describes the location of the atoms, not the electrons. the. What Is Electronic Geometry.
From jeryrecovery.weebly.com
Electron geometry chart 8 electron groups jeryrecovery What Is Electronic Geometry molecular structure describes the location of the atoms, not the electrons. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. The. What Is Electronic Geometry.
From scientifictutor.org
Chem College Electron Geometry and Steric Number Scientific Tutor What Is Electronic Geometry We differentiate between these two situations by naming. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the main difference between electron geometry and molecular geometry is that electron geometry is found by. molecular structure describes the location of the atoms, not the electrons. Bonded atoms and unshared. What Is Electronic Geometry.
From arturowbryant.github.io
Molecular Geometry And Electron Geometry Chart What Is Electronic Geometry The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. the main difference between electron geometry and molecular geometry is that electron geometry is found by. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. molecular structure describes. What Is Electronic Geometry.
From tiklorise.weebly.com
Electron geometry chart tiklorise What Is Electronic Geometry the main difference between electron geometry and molecular geometry is that electron geometry is found by. We differentiate between these two situations by naming. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. The vsepr theory detremines molecular geometries (linear,. What Is Electronic Geometry.
From chartwalls.blogspot.com
Electron Pair Geometry And Molecular Geometry Chart Chart Walls What Is Electronic Geometry The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the main difference between electron geometry and molecular geometry is. What Is Electronic Geometry.
From www.differencebetween.net
Difference Between Electron Geometry and Molecular Geometry What Is Electronic Geometry molecular structure describes the location of the atoms, not the electrons. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. We differentiate between these two situations by naming. the premise of the vsepr theory is that electron. What Is Electronic Geometry.
From www.youtube.com
How to Determine Electron Geometry and Molecular Geometry & Shape with What Is Electronic Geometry Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. the main difference between electron geometry and molecular geometry is that electron. What Is Electronic Geometry.
From proquestyamaha.web.fc2.com
What is the electron geometry of SO3? What Is Electronic Geometry Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. We differentiate between these two situations by naming. after calculating the electronic. What Is Electronic Geometry.
From pediaa.com
Difference Between Electron Geometry and Molecular Geometry What Is Electronic Geometry Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. We differentiate between these two situations by naming. the premise. What Is Electronic Geometry.
From www.slideserve.com
PPT CHM 2045 Molecular Geometry & Chemical Bonding Chapter 10 What Is Electronic Geometry the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. the main difference between electron geometry and molecular geometry is that electron geometry is found by. Bonded atoms and unshared pairs of electrons about a central atom are as far from. What Is Electronic Geometry.
From wisc.pb.unizin.org
Predicting Molecular Shapes VSEPR Model (M9Q1) UWMadison Chemistry What Is Electronic Geometry the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. the main difference between electron geometry and molecular geometry is that electron geometry is found by. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. Bonded atoms and unshared. What Is Electronic Geometry.
From www.coursehero.com
[Solved] please solve What is the correct electronic geometry and What Is Electronic Geometry Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. after calculating the electronic geometry from vespr we can determine the molecular geometry based on the bonding orbitals. the main difference between electron geometry and molecular geometry is that electron geometry is found by. We differentiate between these two. What Is Electronic Geometry.
From slkoti.weebly.com
slkoti Blog What Is Electronic Geometry the premise of the vsepr theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places. The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. molecular. What Is Electronic Geometry.
From chloebarrett.z13.web.core.windows.net
Electron Domain Geometry Chart What Is Electronic Geometry The vsepr theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral,. Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. molecular structure describes the location of the atoms, not the electrons. We differentiate between these two situations by naming. after calculating the electronic geometry from vespr we can. What Is Electronic Geometry.