How To Calculate Q Water . 200 gpm water enters a coil at 120°f and leaves at 80°f. The specific heat capacity of water is 4.18 j/g/°c; \scriptsize \delta q = m \cdot c \cdot. The temperature of the water rose by 30 o c. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. The heat energy change, q, can be calculated by: Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. Q = the heat energy. How to calculate the sensible heat transfer of water. Constant volume calorimetry, also know as bomb calorimetry, is used to. The equation looks like this: Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. In a calorimeter, we can measure temperature change with a thermometer. This equation binds temperature change and heat:
from www.chegg.com
Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. The temperature of the water rose by 30 o c. The heat energy change, q, can be calculated by: Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The specific heat capacity of water is 4.18 j/g/°c; How to calculate the sensible heat transfer of water. \scriptsize \delta q = m \cdot c \cdot. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. The equation looks like this: 200 gpm water enters a coil at 120°f and leaves at 80°f.
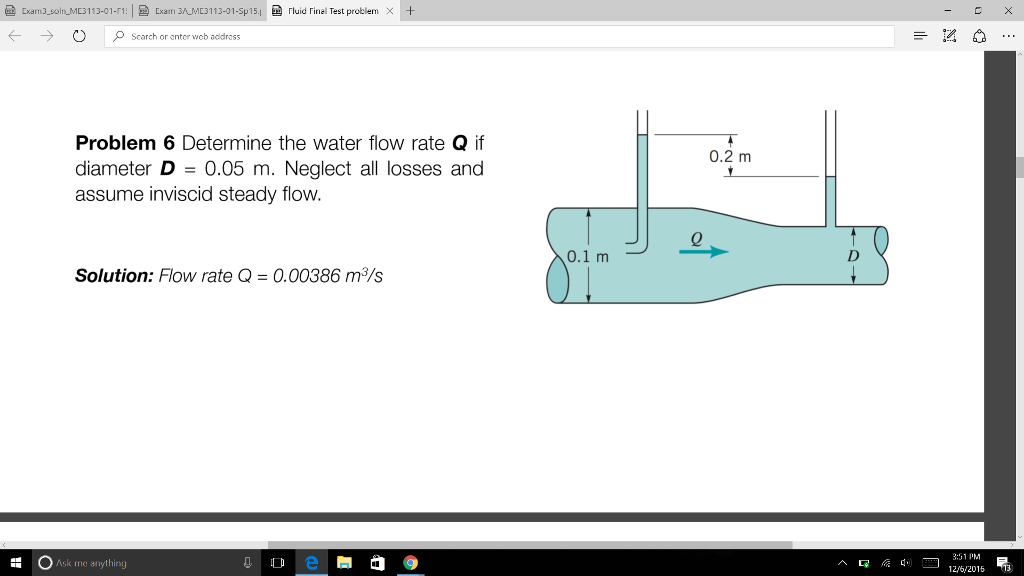
Solved Determine the water flow rate Q if diameter D = 0.05
How To Calculate Q Water How to calculate the sensible heat transfer of water. Constant volume calorimetry, also know as bomb calorimetry, is used to. The equation looks like this: The heat energy change, q, can be calculated by: In a calorimeter, we can measure temperature change with a thermometer. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. How to calculate the sensible heat transfer of water. 200 gpm water enters a coil at 120°f and leaves at 80°f. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The specific heat capacity of water is 4.18 j/g/°c; The temperature of the water rose by 30 o c. Q = the heat energy. Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. \scriptsize \delta q = m \cdot c \cdot. This equation binds temperature change and heat:
From www.youtube.com
Assuming the water vapor to be a perfect gas, calculate the internal How To Calculate Q Water The equation looks like this: 200 gpm water enters a coil at 120°f and leaves at 80°f. The heat energy change, q, can be calculated by: This equation binds temperature change and heat: In a calorimeter, we can measure temperature change with a thermometer. Q = mass x δt x specific heat (water) note that the number of calories and. How To Calculate Q Water.
From www.tessshebaylo.com
Chemical Equation For Water Evaporation Tessshebaylo How To Calculate Q Water 200 gpm water enters a coil at 120°f and leaves at 80°f. Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. Q = the heat energy. Constant volume calorimetry, also know as bomb calorimetry, is used to. Describe a simple calorimeter and explain how it is employed and. How To Calculate Q Water.
From www.tgfitness.com
Water Intake Calculator How To Calculate Q Water The temperature of the water rose by 30 o c. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. In a calorimeter, we can measure temperature change with a thermometer. Q = the heat energy. Describe a simple calorimeter and explain how it is employed and how its heat capacity. How To Calculate Q Water.
From www.youtube.com
Thermochemistry Water Phase Change Heat Calculation.wmv YouTube How To Calculate Q Water How to calculate the sensible heat transfer of water. The equation looks like this: \scriptsize \delta q = m \cdot c \cdot. The temperature of the water rose by 30 o c. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. In a calorimeter, we can measure temperature change with. How To Calculate Q Water.
From haipernews.com
How To Calculate Joules Released Haiper How To Calculate Q Water 200 gpm water enters a coil at 120°f and leaves at 80°f. Constant volume calorimetry, also know as bomb calorimetry, is used to. In a calorimeter, we can measure temperature change with a thermometer. The heat energy change, q, can be calculated by: This equation binds temperature change and heat: Describe a simple calorimeter and explain how it is employed. How To Calculate Q Water.
From serc.carleton.edu
Exercise to Calculate River Discharge How To Calculate Q Water 200 gpm water enters a coil at 120°f and leaves at 80°f. Constant volume calorimetry, also know as bomb calorimetry, is used to. The equation looks like this: The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. Q = mass x δt x specific heat (water) note that the number. How To Calculate Q Water.
From www.chegg.com
Heat of vaporization of water at 100degree C and How To Calculate Q Water The equation looks like this: Q = the heat energy. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. How to calculate the sensible heat transfer of water. Constant volume calorimetry, also know as bomb calorimetry, is used to. The temperature of the water rose by 30 o c. The heat energy. How To Calculate Q Water.
From www.inchcalculator.com
Water Velocity Calculator Inch Calculator How To Calculate Q Water Q = the heat energy. Constant volume calorimetry, also know as bomb calorimetry, is used to. 200 gpm water enters a coil at 120°f and leaves at 80°f. The temperature of the water rose by 30 o c. How to calculate the sensible heat transfer of water. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants. How To Calculate Q Water.
From www.nsrwa.org
How Much Water Do YOU Use? « North & South Rivers Watershed Association How To Calculate Q Water The temperature of the water rose by 30 o c. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. 200 gpm water enters a coil at 120°f and leaves at 80°f. How to calculate the sensible heat transfer of water. Constant volume calorimetry, also know as bomb calorimetry, is used. How To Calculate Q Water.
From www.showme.com
Solving q=mcat Science, Chemistry ShowMe How To Calculate Q Water The specific heat capacity of water is 4.18 j/g/°c; 200 gpm water enters a coil at 120°f and leaves at 80°f. The equation looks like this: Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. Q = the heat energy. Constant volume calorimetry, also know as bomb calorimetry,. How To Calculate Q Water.
From animalia-life.club
Calculator How Much Water How To Calculate Q Water Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. 200 gpm water enters a coil at 120°f and leaves at 80°f. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. The heat energy change, q, can be calculated by: Constant volume calorimetry,. How To Calculate Q Water.
From www.youtube.com
Finding equilibrium concentrations using Q YouTube How To Calculate Q Water The temperature of the water rose by 30 o c. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. How to calculate the sensible heat transfer of water. The specific heat capacity of water is 4.18 j/g/°c; The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during. How To Calculate Q Water.
From labquiz.netlify.app
Water treatment mathematical formulas labquiz How To Calculate Q Water The temperature of the water rose by 30 o c. Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. The specific heat capacity of water is 4.18 j/g/°c; The heat energy change, q, can be calculated by: Describe a simple calorimeter and explain how it is employed and. How To Calculate Q Water.
From calculator.academy
Bottled Water Cost Calculator Calculator Academy How To Calculate Q Water The equation looks like this: Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. Q = the heat energy. 200 gpm water enters a coil at 120°f and leaves at 80°f. The specific. How To Calculate Q Water.
From www.wikihow.com
How to Calculate Water Pump Horsepower 14 Steps (with Pictures) How To Calculate Q Water This equation binds temperature change and heat: Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. The specific heat capacity of water is 4.18 j/g/°c; 200 gpm water enters a coil. How To Calculate Q Water.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to How To Calculate Q Water Constant volume calorimetry, also know as bomb calorimetry, is used to. How to calculate the sensible heat transfer of water. \scriptsize \delta q = m \cdot c \cdot. The heat energy change, q, can be calculated by: Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. This equation binds temperature change and. How To Calculate Q Water.
From calconcalculator.com
Total Body Water Calculator Formula Definition How To Calculate Q Water Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. The specific heat capacity of water is 4.18 j/g/°c; The heat energy change, q, can be calculated by: The temperature of the water rose by 30 o c. Q = the heat energy. The reaction quotient (\ (q\)) measures. How To Calculate Q Water.
From haipernews.com
How To Find Q Equilibrium Constant Haiper How To Calculate Q Water The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. 200 gpm water enters a coil at 120°f and leaves at 80°f. The equation looks like this: This equation binds temperature change and heat: Q = mass x δt x specific heat (water) note that the number of calories and joules. How To Calculate Q Water.
From www.chegg.com
Solved Determine the water flow rate Q if diameter D = 0.05 How To Calculate Q Water The specific heat capacity of water is 4.18 j/g/°c; 200 gpm water enters a coil at 120°f and leaves at 80°f. The equation looks like this: The temperature of the water rose by 30 o c. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. How to calculate the sensible heat transfer. How To Calculate Q Water.
From thechemistrynotes.com
Reaction Quotient (Q) Equation, Calculation, Types, Units How To Calculate Q Water In a calorimeter, we can measure temperature change with a thermometer. The specific heat capacity of water is 4.18 j/g/°c; 200 gpm water enters a coil at 120°f and leaves at 80°f. \scriptsize \delta q = m \cdot c \cdot. Constant volume calorimetry, also know as bomb calorimetry, is used to. Q = the heat energy. The reaction quotient (\. How To Calculate Q Water.
From www.youtube.com
Using the formula q=mcΔT (Three examples) YouTube How To Calculate Q Water The specific heat capacity of water is 4.18 j/g/°c; Q = the heat energy. The equation looks like this: Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. The heat energy change, q,. How To Calculate Q Water.
From www.enieboss.co
calculate the reaction quotient reaction quotient calculator How To Calculate Q Water Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. This equation binds temperature change and heat: How to calculate the sensible heat transfer of water. \scriptsize \delta q = m \cdot c \cdot. Constant volume calorimetry, also know as bomb calorimetry, is used to. 200 gpm water enters. How To Calculate Q Water.
From haipernews.com
How To Calculate Heat Capacity Of Water Haiper How To Calculate Q Water The equation looks like this: This equation binds temperature change and heat: The heat energy change, q, can be calculated by: Constant volume calorimetry, also know as bomb calorimetry, is used to. How to calculate the sensible heat transfer of water. \scriptsize \delta q = m \cdot c \cdot. Describe a simple calorimeter and explain how it is employed and. How To Calculate Q Water.
From in.pinterest.com
Q/A 292 HOW TO CALCULATE WATER QUANTITY FOR CONCRETE Civil How To Calculate Q Water The heat energy change, q, can be calculated by: In a calorimeter, we can measure temperature change with a thermometer. Constant volume calorimetry, also know as bomb calorimetry, is used to. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The specific heat capacity of water is 4.18 j/g/°c; This equation binds. How To Calculate Q Water.
From chemguru.sg
How to Calculate pH of Water How To Calculate Q Water Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. The equation looks like this: The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. Constant volume calorimetry, also know as bomb calorimetry, is used to. In a calorimeter, we. How To Calculate Q Water.
From www.researchgate.net
Standard curve established for MilliQ water base and surface water How To Calculate Q Water 200 gpm water enters a coil at 120°f and leaves at 80°f. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. The temperature of the water rose by 30 o c. Q = the heat energy. In a calorimeter, we can measure temperature change with a thermometer. This equation binds. How To Calculate Q Water.
From www.bartleby.com
Answered Expert solution/answer Specific heat of… bartleby How To Calculate Q Water \scriptsize \delta q = m \cdot c \cdot. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. The equation looks like this: Q = the heat energy. Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. How to. How To Calculate Q Water.
From haipernews.com
How To Find Q Equilibrium Constant Haiper How To Calculate Q Water The equation looks like this: Q = the heat energy. In a calorimeter, we can measure temperature change with a thermometer. The heat energy change, q, can be calculated by: The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. Q = mass x δt x specific heat (water) note that. How To Calculate Q Water.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube How To Calculate Q Water \scriptsize \delta q = m \cdot c \cdot. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. In a calorimeter, we can measure temperature change with a thermometer. The equation looks like this: The heat energy change, q, can be calculated by: How to calculate the sensible heat transfer of water. 200. How To Calculate Q Water.
From haipernews.com
How To Calculate Q From Kc Haiper How To Calculate Q Water This equation binds temperature change and heat: In a calorimeter, we can measure temperature change with a thermometer. Q = the heat energy. How to calculate the sensible heat transfer of water. The heat energy change, q, can be calculated by: Constant volume calorimetry, also know as bomb calorimetry, is used to. 200 gpm water enters a coil at 120°f. How To Calculate Q Water.
From www.youtube.com
4 Q value calculations YouTube How To Calculate Q Water The temperature of the water rose by 30 o c. 200 gpm water enters a coil at 120°f and leaves at 80°f. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Constant volume calorimetry, also know as bomb calorimetry, is used to. Q = the heat energy. \scriptsize \delta q = m. How To Calculate Q Water.
From partdiagrambonvipt.z21.web.core.windows.net
How To Calculate Water Pump Hp How To Calculate Q Water Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. How to calculate the sensible heat transfer of water. 200 gpm water enters a coil at 120°f and leaves at 80°f. The equation looks like this: The temperature of the water rose by 30 o c. In a calorimeter,. How To Calculate Q Water.
From sractclx.blogspot.com
Specific Heat Capacity Water / schoolphysics The specific How To Calculate Q Water The equation looks like this: The specific heat capacity of water is 4.18 j/g/°c; The heat energy change, q, can be calculated by: Q = mass x δt x specific heat (water) note that the number of calories and joules may seem high but. In a calorimeter, we can measure temperature change with a thermometer. The temperature of the water. How To Calculate Q Water.
From animalia-life.club
Calculator How Much Water How To Calculate Q Water Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The heat energy change, q, can be calculated by: 200 gpm water enters a coil at 120°f and leaves at 80°f. In a calorimeter, we can measure temperature change with a thermometer. Constant volume calorimetry, also know as bomb calorimetry, is used to.. How To Calculate Q Water.
From www.youtube.com
AP Chemistry Calculating Q and Equilibrium Compositions YouTube How To Calculate Q Water The equation looks like this: The heat energy change, q, can be calculated by: \scriptsize \delta q = m \cdot c \cdot. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The reaction quotient (\ (q\)) measures the relative amounts of products and reactants present during a reaction at a. The specific. How To Calculate Q Water.