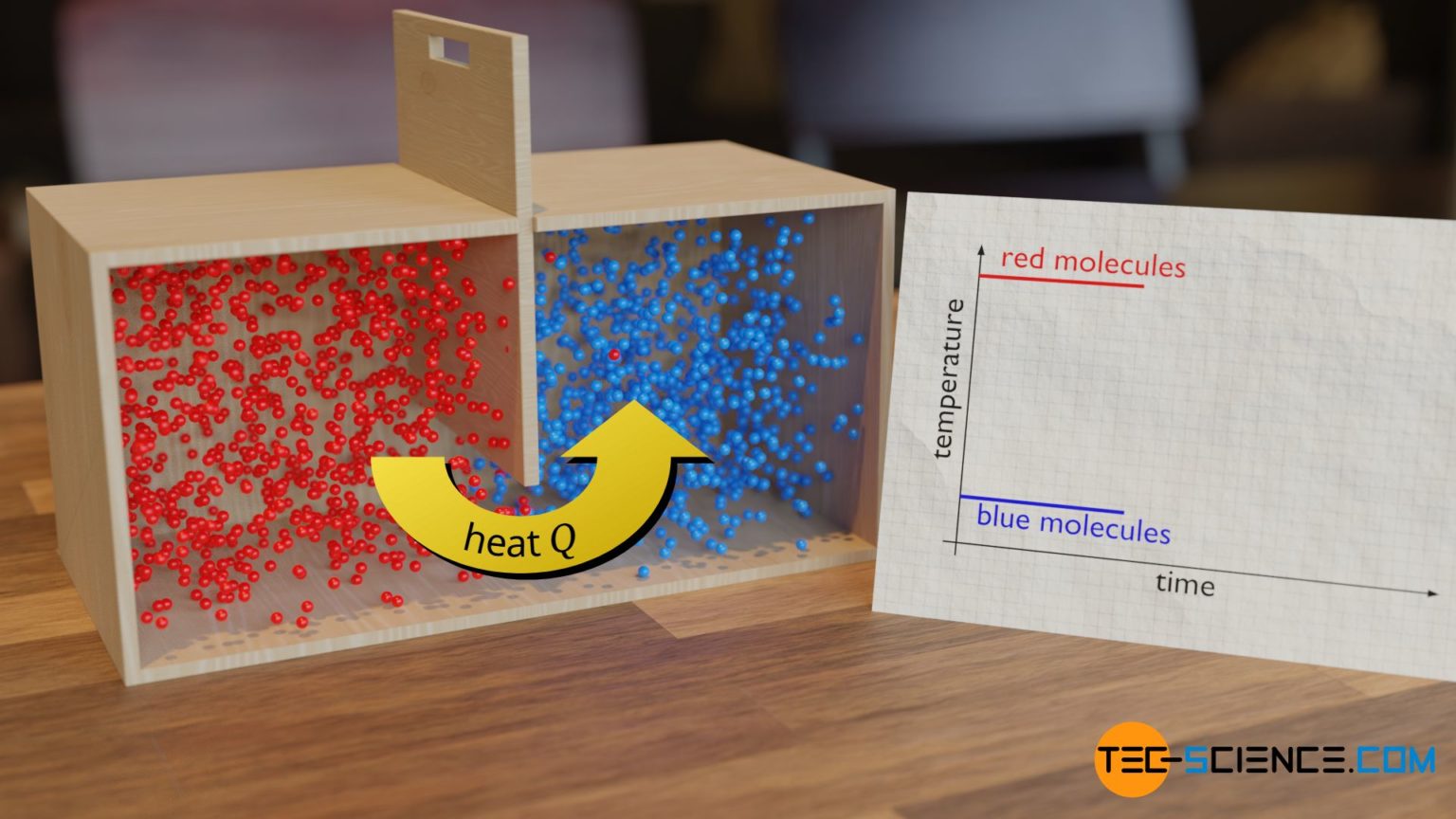
Thermal Equilibrium Coffee . Energy is transferred by heating from the hot coffee to the cold surroundings. In the case of your hot coffee in. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. Cooling a cup of coffee. Various cooling strategies demonstrate specific heat,. When the objects reach the same temperature as the surrounding. You have a 200 gram cup of coffee at 100 °c, too hot to drink. This causes the coffee to cool down. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy).
from www.tec-science.com
Cooling a cup of coffee. In the case of your hot coffee in. This causes the coffee to cool down. Energy is transferred by heating from the hot coffee to the cold surroundings. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). When the objects reach the same temperature as the surrounding. You have a 200 gram cup of coffee at 100 °c, too hot to drink. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer.
Heat and thermodynamic equilibrium tecscience
Thermal Equilibrium Coffee Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. Energy is transferred by heating from the hot coffee to the cold surroundings. You have a 200 gram cup of coffee at 100 °c, too hot to drink. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. When the objects reach the same temperature as the surrounding. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Various cooling strategies demonstrate specific heat,. Cooling a cup of coffee. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. This causes the coffee to cool down. In the case of your hot coffee in. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy).
From www.vedantu.com
Thermal Equilibrium Types and Mechanism for JEE Thermal Equilibrium Coffee You have a 200 gram cup of coffee at 100 °c, too hot to drink. Cooling a cup of coffee. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Energy is transferred by heating from the hot coffee to the cold surroundings. Thermal equilibrium is a. Thermal Equilibrium Coffee.
From www.dreamstime.com
Fourier S Law and Heat Transfer Process through a Materials Stock Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). You have a 200 gram cup of coffee at 100 °c, too hot to drink. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. If it is defined that a thermodynamic system is. Thermal Equilibrium Coffee.
From favpng.com
Heat Thermal Equilibrium Physics Temperature Measurement Research, PNG Thermal Equilibrium Coffee Energy is transferred by heating from the hot coffee to the cold surroundings. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Cooling a cup of coffee. In the case of your hot coffee in. Entropy is a measure of whether energy is localized (low entropy) or dispersed. Thermal Equilibrium Coffee.
From brainly.in
an example of thermal equilibrium Brainly.in Thermal Equilibrium Coffee Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. In. Thermal Equilibrium Coffee.
From www.fantine.io
Thermal Shock in Coffee 8 Things You Should Know Fantine Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). Various cooling strategies demonstrate specific heat,. You have a 200 gram cup of coffee at 100 °c, too hot to drink. This causes the coffee to cool down. When the objects reach the same temperature as the surrounding. In the case of your hot coffee. Thermal Equilibrium Coffee.
From psiberg.com
Zeroth Law of Thermodynamics The Thermal Equilibrium Law PSIBERG Thermal Equilibrium Coffee The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). You have a 200 gram cup of coffee at 100 °c, too hot to drink. Thermal equilibrium is a state in which two or. Thermal Equilibrium Coffee.
From gamesmartz.com
Thermal Equilibrium Definition & Image GameSmartz Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). Energy is transferred by heating from the hot coffee to the cold surroundings. Cooling a cup of coffee. This causes the coffee to cool down. You have a 200 gram cup of coffee at 100 °c, too hot to drink. When the objects reach the. Thermal Equilibrium Coffee.
From www.dreamstime.com
Fourier S Law and Heat Transfer Process through a Materials Via Direct Thermal Equilibrium Coffee Various cooling strategies demonstrate specific heat,. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Energy is transferred by heating from the hot coffee to the cold surroundings. You have a 200 gram cup of coffee at 100 °c, too hot to drink. In the case of your. Thermal Equilibrium Coffee.
From www.tekportal.net
thermal equilibrium Liberal Dictionary Thermal Equilibrium Coffee Energy is transferred by heating from the hot coffee to the cold surroundings. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). You have a 200 gram cup of coffee at 100 °c, too hot to drink. Cooling a cup of coffee. Thermal equilibrium is a state in which two or more bodies (or. Thermal Equilibrium Coffee.
From learnbin.net
The Zeroth Law Of Thermodynamics The Concept Of Temperature Learnbin Thermal Equilibrium Coffee You have a 200 gram cup of coffee at 100 °c, too hot to drink. Cooling a cup of coffee. When the objects reach the same temperature as the surrounding. Energy is transferred by heating from the hot coffee to the cold surroundings. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature. Thermal Equilibrium Coffee.
From www.worksheetsplanet.com
What is Thermal Equilibrium Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. This causes the coffee to cool down. Cooling a cup of coffee. Various cooling strategies demonstrate specific heat,. Energy is transferred by heating from the hot. Thermal Equilibrium Coffee.
From www.geeksforgeeks.org
What is Thermodynamics Definition, Laws, Formulas, Class 11 Notes Thermal Equilibrium Coffee Energy is transferred by heating from the hot coffee to the cold surroundings. This causes the coffee to cool down. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Various cooling strategies demonstrate specific heat,. When the objects reach the same temperature as the surrounding. Entropy is a. Thermal Equilibrium Coffee.
From stock.adobe.com
Zeroth Law of Thermodynamics Infographic Diagram showing three systems Thermal Equilibrium Coffee This causes the coffee to cool down. You have a 200 gram cup of coffee at 100 °c, too hot to drink. Cooling a cup of coffee. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. In the case of your hot coffee in. Various cooling strategies demonstrate. Thermal Equilibrium Coffee.
From storables.com
How To Store Thermal Paste Storables Thermal Equilibrium Coffee Energy is transferred by heating from the hot coffee to the cold surroundings. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). In the case of your hot coffee in. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Various. Thermal Equilibrium Coffee.
From www.dreamstime.com
Heat Flow between Substances Stock Vector Illustration of blue Thermal Equilibrium Coffee Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Various cooling strategies demonstrate specific heat,. When the objects reach the same temperature as the surrounding. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. You have. Thermal Equilibrium Coffee.
From www.studypool.com
SOLUTION What is thermal equilibrium physics Studypool Thermal Equilibrium Coffee You have a 200 gram cup of coffee at 100 °c, too hot to drink. Energy is transferred by heating from the hot coffee to the cold surroundings. Cooling a cup of coffee. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. This causes the coffee to cool. Thermal Equilibrium Coffee.
From www.chegg.com
Solved 6 Equilibrium conditions (8 points). We've spent some Thermal Equilibrium Coffee Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. Energy is transferred by heating from the hot coffee to the cold surroundings. Cooling a cup of coffee. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). You have a 200 gram cup of coffee. Thermal Equilibrium Coffee.
From www.studocu.com
Thermal Equilibrium 11 Thermal Equilibrium Operationaldot tells you Thermal Equilibrium Coffee You have a 200 gram cup of coffee at 100 °c, too hot to drink. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Cooling a cup of coffee. When the objects reach the same temperature as the surrounding. Entropy is a measure of whether energy is localized. Thermal Equilibrium Coffee.
From www.tessshebaylo.com
Final Equilibrium Temperature Equation Tessshebaylo Thermal Equilibrium Coffee Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. In the case of your hot coffee in. When the objects reach the same temperature as the surrounding. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. You have. Thermal Equilibrium Coffee.
From www.tec-science.com
Heat and thermodynamic equilibrium tecscience Thermal Equilibrium Coffee If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Cooling a cup of coffee. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. This causes the coffee to cool down. Various cooling strategies demonstrate. Thermal Equilibrium Coffee.
From pandai.me
Thermal Equilibrium Thermal Equilibrium Coffee In the case of your hot coffee in. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Energy is transferred by heating from the hot coffee to the cold surroundings. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). Various. Thermal Equilibrium Coffee.
From www.chegg.com
Solved 5. You are given that in thermal equilibrium the mean Thermal Equilibrium Coffee When the objects reach the same temperature as the surrounding. Energy is transferred by heating from the hot coffee to the cold surroundings. You have a 200 gram cup of coffee at 100 °c, too hot to drink. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. Thermal equilibrium is. Thermal Equilibrium Coffee.
From slideplayer.com
Solid Solution Thermal Equilibrium Diagram ppt download Thermal Equilibrium Coffee If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). When. Thermal Equilibrium Coffee.
From thechemistrynotes.com
Thermodynamic Processes The Chemistry Notes Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. This causes the coffee to cool down. Cooling a cup of coffee. When the objects reach the same temperature as the surrounding.. Thermal Equilibrium Coffee.
From www.tec-science.com
Heat and thermodynamic equilibrium tecscience Thermal Equilibrium Coffee Energy is transferred by heating from the hot coffee to the cold surroundings. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). When the objects reach the same temperature as the surrounding. If it is. Thermal Equilibrium Coffee.
From www.youtube.com
Equilibrium Coffee Intertrade Corp. WOFEX 2022 YouTube Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Energy. Thermal Equilibrium Coffee.
From brainly.in
When two objects are said to be in thermal equilibrium? Brainly.in Thermal Equilibrium Coffee Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. Cooling a cup of coffee. Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). This causes the coffee to cool down. The thermal equilibrium calculator helps you determine the final temperature between two elements or. Thermal Equilibrium Coffee.
From www.numerade.com
SOLVED QUESTION 7 The answer to the previous coffee cup question is an Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). Cooling a cup of coffee. In the case of your hot coffee in. Various cooling strategies demonstrate specific heat,. You have a 200 gram cup of coffee at 100 °c, too hot to drink. This causes the coffee to cool down. Energy is transferred by. Thermal Equilibrium Coffee.
From quizizz.com
Thermal Equilibrium 93 plays Quizizz Thermal Equilibrium Coffee Cooling a cup of coffee. In the case of your hot coffee in. Energy is transferred by heating from the hot coffee to the cold surroundings. The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Various cooling strategies demonstrate specific heat,. When the objects reach the same temperature. Thermal Equilibrium Coffee.
From thermalengineeringlearn.blogspot.com
THERMAL ENGINEERING Thermodynamic Equilibrium Thermal Equilibrium Coffee Entropy is a measure of whether energy is localized (low entropy) or dispersed (high entropy). Energy is transferred by heating from the hot coffee to the cold surroundings. This causes the coffee to cool down. Various cooling strategies demonstrate specific heat,. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore. Thermal Equilibrium Coffee.
From quizizz.com
50+ heat transfer and thermal equilibrium worksheets on Quizizz Free Thermal Equilibrium Coffee Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then. Thermal Equilibrium Coffee.
From www.savemyexams.com
Thermal Equilibrium OCR A Level Physics Revision Notes 2017 Thermal Equilibrium Coffee If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and. Thermal Equilibrium Coffee.
From laptopfriendly.co
Equilibrium Kaffee A WorkFriendly Place in Vienna Thermal Equilibrium Coffee When the objects reach the same temperature as the surrounding. Thermal equilibrium is a state in which two or more bodies (or systems) reach the same temperature and therefore no longer. Various cooling strategies demonstrate specific heat,. This causes the coffee to cool down. In the case of your hot coffee in. The thermal equilibrium calculator helps you determine the. Thermal Equilibrium Coffee.
From www.studypool.com
SOLUTION What is Thermal equilibrium with practice exercises Thermal Equilibrium Coffee You have a 200 gram cup of coffee at 100 °c, too hot to drink. If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law. When the objects reach the same temperature as the surrounding. Entropy is a measure of whether energy is localized (low entropy) or. Thermal Equilibrium Coffee.
From www.studypool.com
SOLUTION Heat Temperature And Thermal Equilibrium Lab Studypool Thermal Equilibrium Coffee The thermal equilibrium calculator helps you determine the final temperature between two elements or systems that are transferring heat between them. Various cooling strategies demonstrate specific heat,. In the case of your hot coffee in. Cooling a cup of coffee. Energy is transferred by heating from the hot coffee to the cold surroundings. Thermal equilibrium is established when two bodies. Thermal Equilibrium Coffee.