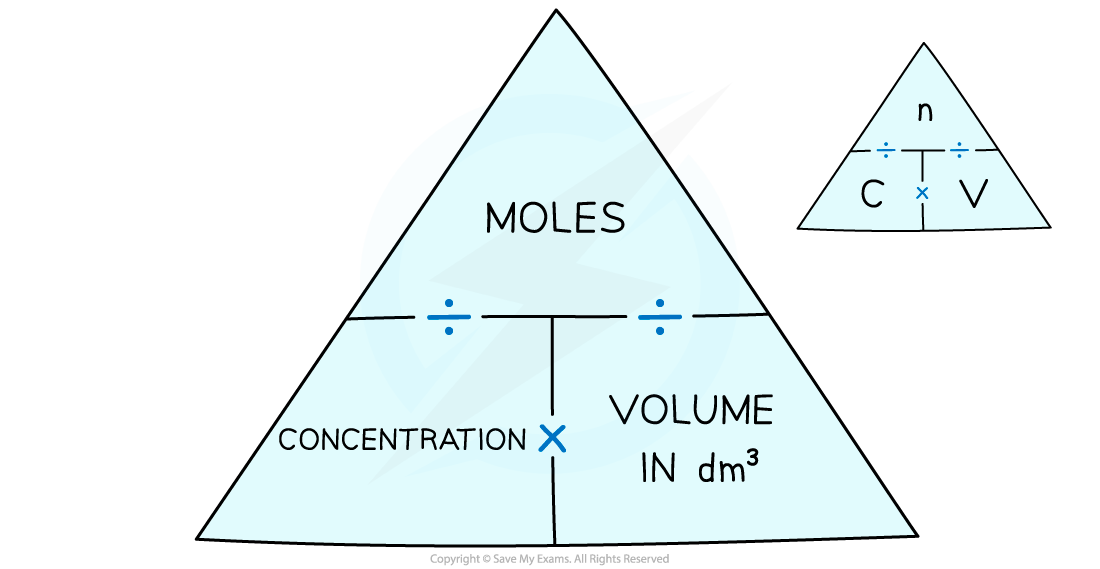
How To Calculate Solvent Concentration . There are a number of ways to express the relative amounts of solute and solvent in a solution. Convert grams of solute and solvent to moles of solute and solvent. You can calculate the concentration of a solution following a dilution by applying this equation: This page describes calculations for. Which unit you use depends on. There are multiple units of concentration. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Calculate the mole fraction of solute by dividing the moles of solute by the. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. There are several ways to express the amount of solute present in a solution. The concentration of a solution is a measure of the amount of solute that has been dissolved in a. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or.
from www.linstitute.net
There are a number of ways to express the relative amounts of solute and solvent in a solution. Convert grams of solute and solvent to moles of solute and solvent. There are multiple units of concentration. Which unit you use depends on. The concentration of a solution is a measure of the amount of solute that has been dissolved in a. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. You can calculate the concentration of a solution following a dilution by applying this equation: In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. Calculate the mole fraction of solute by dividing the moles of solute by the. There are several ways to express the amount of solute present in a solution.
Edexcel IGCSE Chemistry 复习笔记 1.5.9 Calculate Concentrations of
How To Calculate Solvent Concentration Calculate the mole fraction of solute by dividing the moles of solute by the. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. This page describes calculations for. There are several ways to express the amount of solute present in a solution. Convert grams of solute and solvent to moles of solute and solvent. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Calculate the mole fraction of solute by dividing the moles of solute by the. You can calculate the concentration of a solution following a dilution by applying this equation: There are multiple units of concentration. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. There are a number of ways to express the relative amounts of solute and solvent in a solution. The concentration of a solution is a measure of the amount of solute that has been dissolved in a.
From www.youtube.com
How to Calculate Mass Percent of Solute and Solvent of Solution How To Calculate Solvent Concentration There are several ways to express the amount of solute present in a solution. This page describes calculations for. There are multiple units of concentration. Which unit you use depends on. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. The concentration of a solution is. How To Calculate Solvent Concentration.
From www.pinterest.com
How to Calculate the Concentration of a Solution Solutions How To Calculate Solvent Concentration Calculate the mole fraction of solute by dividing the moles of solute by the. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. Which unit you use depends on. This page describes calculations for. You can calculate the concentration of a solution following a dilution by. How To Calculate Solvent Concentration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Solvent Concentration The concentration of a solution is a measure of the amount of solute that has been dissolved in a. Calculate the mole fraction of solute by dividing the moles of solute by the. There are multiple units of concentration. Which unit you use depends on. There are a number of ways to express the relative amounts of solute and solvent. How To Calculate Solvent Concentration.
From www.youtube.com
Solubility vs Concentration Basic Introduction, Saturated Unsaturated How To Calculate Solvent Concentration Calculate the mole fraction of solute by dividing the moles of solute by the. You can calculate the concentration of a solution following a dilution by applying this equation: Convert grams of solute and solvent to moles of solute and solvent. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. There are. How To Calculate Solvent Concentration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Solvent Concentration There are a number of ways to express the relative amounts of solute and solvent in a solution. There are multiple units of concentration. Which unit you use depends on. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. You can calculate the concentration of a solution following a dilution by applying. How To Calculate Solvent Concentration.
From lacifersbernard.blogspot.com
How to Calculate Which Is the Best Solvent How To Calculate Solvent Concentration Convert grams of solute and solvent to moles of solute and solvent. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. You can calculate the concentration of a solution following a dilution by applying this equation: The concentration of a solution is a measure of the amount of solute that has been. How To Calculate Solvent Concentration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Solvent Concentration Convert grams of solute and solvent to moles of solute and solvent. This page describes calculations for. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Calculate the mole fraction of solute by dividing the moles of solute by the. M i v i = m f v f where m is. How To Calculate Solvent Concentration.
From www.pdfprof.com
mass concentration calculator How To Calculate Solvent Concentration The concentration of a solution is a measure of the amount of solute that has been dissolved in a. This page describes calculations for. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. Calculate the mole fraction of solute by dividing the moles of solute by. How To Calculate Solvent Concentration.
From www.yaclass.in
Calculation Concentration of the solution — lesson. Science CBSE, Class 9. How To Calculate Solvent Concentration Calculate the mole fraction of solute by dividing the moles of solute by the. The concentration of a solution is a measure of the amount of solute that has been dissolved in a. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Concentration is an expression. How To Calculate Solvent Concentration.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii How To Calculate Solvent Concentration In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. There are several ways to express the amount of solute present in a solution.. How To Calculate Solvent Concentration.
From masterconceptsinchemistry.com
How to calculate solution concentration in mass percent How To Calculate Solvent Concentration This page describes calculations for. Convert grams of solute and solvent to moles of solute and solvent. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. The concentration of a solution is a measure of the amount of solute that has been dissolved in a. Calculate the mole fraction of solute by. How To Calculate Solvent Concentration.
From www.youtube.com
1.3 Concentration of solutions YouTube How To Calculate Solvent Concentration The concentration of a solution is a measure of the amount of solute that has been dissolved in a. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. There are a number of ways to express the relative amounts of solute and solvent in a solution.. How To Calculate Solvent Concentration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Solvent Concentration There are multiple units of concentration. Calculate the mole fraction of solute by dividing the moles of solute by the. The concentration of a solution is a measure of the amount of solute that has been dissolved in a. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of. How To Calculate Solvent Concentration.
From www.youtube.com
1.3 Distinguish between solute, solvent, solution and concentration [SL How To Calculate Solvent Concentration Convert grams of solute and solvent to moles of solute and solvent. The concentration of a solution is a measure of the amount of solute that has been dissolved in a. There are a number of ways to express the relative amounts of solute and solvent in a solution. You can calculate the concentration of a solution following a dilution. How To Calculate Solvent Concentration.
From www.nagwa.com
Question Video Identifying the Equation Used to Calculate the How To Calculate Solvent Concentration There are several ways to express the amount of solute present in a solution. Calculate the mole fraction of solute by dividing the moles of solute by the. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. In chemistry, the concentration of a solution describes the. How To Calculate Solvent Concentration.
From socratic.org
mL of solution has a concentration of 3.0 M. How much water needs to be How To Calculate Solvent Concentration There are multiple units of concentration. Convert grams of solute and solvent to moles of solute and solvent. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Which unit you use depends on. The concentration of a solution is a measure of the amount of solute. How To Calculate Solvent Concentration.
From www.youtube.com
Equilibrium Concentrations (Example) YouTube How To Calculate Solvent Concentration Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. There are multiple units of concentration. Convert grams of solute and solvent to moles of solute and solvent. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular. How To Calculate Solvent Concentration.
From chemconnections.org
Solution Concentration How To Calculate Solvent Concentration There are several ways to express the amount of solute present in a solution. The concentration of a solution is a measure of the amount of solute that has been dissolved in a. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Convert grams of solute and solvent to moles of solute. How To Calculate Solvent Concentration.
From www.youtube.com
Calculating the Concentration of a Standardized Solution YouTube How To Calculate Solvent Concentration Convert grams of solute and solvent to moles of solute and solvent. You can calculate the concentration of a solution following a dilution by applying this equation: Calculate the mole fraction of solute by dividing the moles of solute by the. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. This page. How To Calculate Solvent Concentration.
From nl.wikihow.com
De concentratie van een oplossing bepalen wikiHow How To Calculate Solvent Concentration Which unit you use depends on. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. There are a number of ways to express the relative amounts of solute and solvent in a solution. M i v i = m f v f where m is molarity,. How To Calculate Solvent Concentration.
From www.youtube.com
1.3 Solve Problems Using Concentration, Amount of Solute and Volume [SL How To Calculate Solvent Concentration You can calculate the concentration of a solution following a dilution by applying this equation: There are multiple units of concentration. Convert grams of solute and solvent to moles of solute and solvent. Calculate the mole fraction of solute by dividing the moles of solute by the. Which unit you use depends on. Concentration is an expression of how much. How To Calculate Solvent Concentration.
From www.youtube.com
NMR spectroscopy in easy way Part6 How to select solvents in NMR How To Calculate Solvent Concentration You can calculate the concentration of a solution following a dilution by applying this equation: Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Which unit you use depends on. Convert grams of solute and solvent to moles of solute and solvent. There are multiple units of concentration. The concentration of a. How To Calculate Solvent Concentration.
From educationalrevolution786.blogspot.com
Concentration> Mole fraction How To Calculate Solvent Concentration There are several ways to express the amount of solute present in a solution. There are multiple units of concentration. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Which unit you use depends on. Concentration is an expression of how much solute is dissolved in. How To Calculate Solvent Concentration.
From www.slideserve.com
PPT Assignment 5.06 Solubility and Concentrations PowerPoint How To Calculate Solvent Concentration You can calculate the concentration of a solution following a dilution by applying this equation: The concentration of a solution is a measure of the amount of solute that has been dissolved in a. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. Calculate the mole. How To Calculate Solvent Concentration.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.5.9 Calculate Concentrations of How To Calculate Solvent Concentration In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. Calculate the mole fraction of solute by dividing the moles of solute by the. There are a number of ways to express the relative amounts of solute and solvent in a solution. Concentration is an expression of. How To Calculate Solvent Concentration.
From drcalef.com
13.7 Concentration ppm and ppb How To Calculate Solvent Concentration This page describes calculations for. There are a number of ways to express the relative amounts of solute and solvent in a solution. You can calculate the concentration of a solution following a dilution by applying this equation: There are several ways to express the amount of solute present in a solution. Calculate the mole fraction of solute by dividing. How To Calculate Solvent Concentration.
From www.youtube.com
Calculating mass of solvent from molality YouTube How To Calculate Solvent Concentration Calculate the mole fraction of solute by dividing the moles of solute by the. Convert grams of solute and solvent to moles of solute and solvent. You can calculate the concentration of a solution following a dilution by applying this equation: This page describes calculations for. In chemistry, the concentration of a solution describes the quantity of a solute that. How To Calculate Solvent Concentration.
From chem.libretexts.org
4.3 Solution Concentrations Chemistry LibreTexts How To Calculate Solvent Concentration You can calculate the concentration of a solution following a dilution by applying this equation: In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. There are multiple units of concentration. Convert grams of solute and solvent to moles of solute and solvent. The concentration of a. How To Calculate Solvent Concentration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Solvent Concentration This page describes calculations for. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. You can calculate the concentration of a solution following a dilution by applying this equation: There are multiple units of concentration. Convert grams of solute and solvent to moles of solute and. How To Calculate Solvent Concentration.
From www.slideserve.com
PPT CALCULATING CONCENTRATION OF SOLUTIONS PowerPoint Presentation How To Calculate Solvent Concentration There are several ways to express the amount of solute present in a solution. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. You can calculate the concentration of a solution following a dilution by applying this equation: The concentration of a solution is a measure of the amount of solute that. How To Calculate Solvent Concentration.
From studylib.net
File How To Calculate Solvent Concentration The concentration of a solution is a measure of the amount of solute that has been dissolved in a. Convert grams of solute and solvent to moles of solute and solvent. In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or. M i v i = m. How To Calculate Solvent Concentration.
From saylordotorg.github.io
Solution Concentrations How To Calculate Solvent Concentration The concentration of a solution is a measure of the amount of solute that has been dissolved in a. You can calculate the concentration of a solution following a dilution by applying this equation: There are a number of ways to express the relative amounts of solute and solvent in a solution. Which unit you use depends on. Calculate the. How To Calculate Solvent Concentration.
From www.pdfprof.com
concentration molaire effective formule How To Calculate Solvent Concentration M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. This page describes calculations for. Which unit you use depends on. There are a number of ways to express the relative amounts of solute and solvent in a solution. There are several ways to express the amount. How To Calculate Solvent Concentration.
From studyingworksheets.com
Solution Concentration Worksheet With Answers Studying Worksheets How To Calculate Solvent Concentration The concentration of a solution is a measure of the amount of solute that has been dissolved in a. Convert grams of solute and solvent to moles of solute and solvent. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. There are several ways to express the amount of solute present in. How To Calculate Solvent Concentration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Solvent Concentration There are several ways to express the amount of solute present in a solution. There are a number of ways to express the relative amounts of solute and solvent in a solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Which unit you use depends. How To Calculate Solvent Concentration.