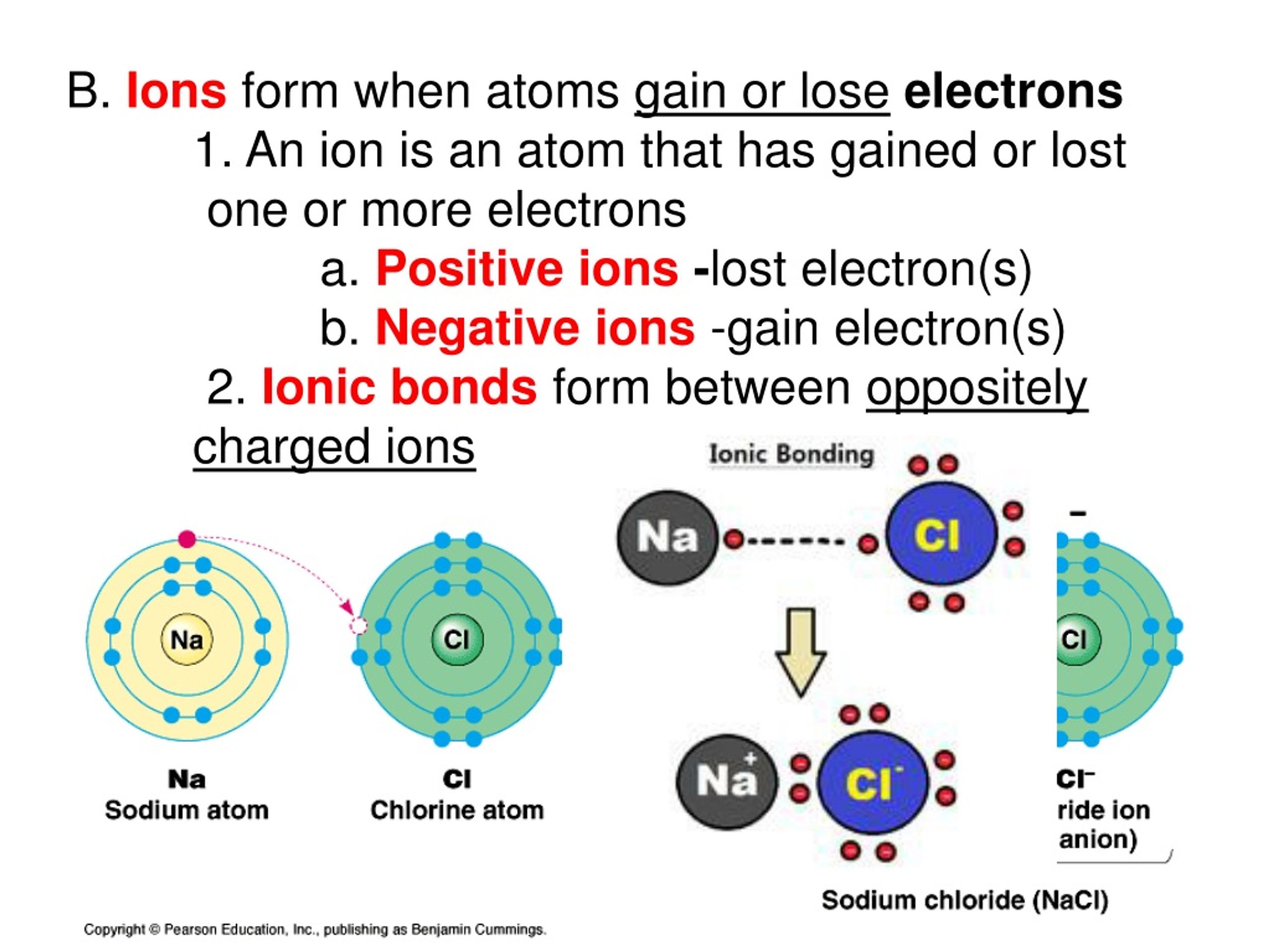
Do Lead Lose Electrons . So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. When a lead atom loses two electrons it exhibits a +2 charge. This causes an increase in metallic character. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually. 93 rows this table shows the most common charges for atoms of the chemical elements. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Hydrogen is an exception, as it will usually lose its electron. You can use this table to predict whether an atom can bond with another atom. In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. Why does it gain electrons when losing them would be. However, elements with a small atomic radius (such as fluorine) will have such a tight.
from www.slideserve.com
During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. However, elements with a small atomic radius (such as fluorine) will have such a tight. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. This causes an increase in metallic character. 93 rows this table shows the most common charges for atoms of the chemical elements. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually. You can use this table to predict whether an atom can bond with another atom. Why does it gain electrons when losing them would be. When a lead atom loses two electrons it exhibits a +2 charge.
PPT UNIT 1 Structure and Function (Biochemistry) Chapter 2
Do Lead Lose Electrons During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually. When a lead atom loses two electrons it exhibits a +2 charge. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. However, elements with a small atomic radius (such as fluorine) will have such a tight. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Why does it gain electrons when losing them would be. Hydrogen is an exception, as it will usually lose its electron. 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can bond with another atom. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. This causes an increase in metallic character. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion.
From twobirdsfourhands.com
Lead Periodic Table Protons Two Birds Home Do Lead Lose Electrons Hydrogen is an exception, as it will usually lose its electron. When a lead atom loses two electrons it exhibits a +2 charge. This causes an increase in metallic character. However, elements with a small atomic radius (such as fluorine) will have such a tight. In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. Atoms tend. Do Lead Lose Electrons.
From www.slideserve.com
PPT UNIT 1 Structure and Function (Biochemistry) Chapter 2 Do Lead Lose Electrons The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Hydrogen is an exception, as it will usually lose its electron. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+).. Do Lead Lose Electrons.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Do Lead Lose Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually. This causes an increase in metallic character. Why does it gain electrons when losing them would be. So, these two electrons will be lost from the last shell, which means electrons will be lost from the. Do Lead Lose Electrons.
From www.alamy.com
Pb Lead, Periodic Table of the Elements, Shell Structure of Lead Do Lead Lose Electrons The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Atoms tend to lose, gain, or share some valance electrons,. Do Lead Lose Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Do Lead Lose Electrons You can use this table to predict whether an atom can bond with another atom. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually. Why does it gain. Do Lead Lose Electrons.
From www.youtube.com
CHEMISTRY 101 Writing an Electron Configuration for Lead Using the Do Lead Lose Electrons During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). 93 rows this table shows the most common charges for atoms of the chemical elements. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. The oxidation state of. Do Lead Lose Electrons.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609 Do Lead Lose Electrons The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Atoms tend to lose, gain, or share some valance electrons,. Do Lead Lose Electrons.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Do Lead Lose Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. So, these two electrons will be lost from the last shell, which means electrons will. Do Lead Lose Electrons.
From www.slideserve.com
PPT PERIODICITY PowerPoint Presentation, free download ID2101289 Do Lead Lose Electrons This causes an increase in metallic character. In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. When a lead atom loses two electrons it exhibits a +2 charge. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in. Do Lead Lose Electrons.
From www.slideserve.com
PPT Ionic and Covalent Bonding PowerPoint Presentation, free download Do Lead Lose Electrons You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. This causes an increase in metallic character. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Atoms tend. Do Lead Lose Electrons.
From awesomehome.co
Lead Periodic Table Electrons Awesome Home Do Lead Lose Electrons The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. 93 rows this table shows the most common charges for atoms of the chemical elements. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Do Lead Lose Electrons.
From www.sciencephoto.com
Lead, atomic structure Stock Image C013/1639 Science Photo Library Do Lead Lose Electrons In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another. Do Lead Lose Electrons.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Image & Art Do Lead Lose Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. You can use this table to predict whether an atom can bond with another atom. So, these two electrons will be lost from the. Do Lead Lose Electrons.
From www.slideserve.com
PPT What causes reactivity of elements PowerPoint Presentation, free Do Lead Lose Electrons Hydrogen is an exception, as it will usually lose its electron. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in. Do Lead Lose Electrons.
From www.slideserve.com
PPT RULES FOR ASSIGNING OXIDATION NUMBERS (STATES PowerPoint Do Lead Lose Electrons Hydrogen is an exception, as it will usually lose its electron. Why does it gain electrons when losing them would be. In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. You can use this table to predict whether an atom can bond with another atom. In general, metals will lose electrons to become a positive cation. Do Lead Lose Electrons.
From www.pinterest.com
Electron Configuration for Pb, Pb2+, and Pb3+ (Lead and Lead Ions Do Lead Lose Electrons This causes an increase in metallic character. Why does it gain electrons when losing them would be. 93 rows this table shows the most common charges for atoms of the chemical elements. However, elements with a small atomic radius (such as fluorine) will have such a tight. So, these two electrons will be lost from the last shell, which means. Do Lead Lose Electrons.
From material-properties.org
Lead Periodic Table and Atomic Properties Do Lead Lose Electrons During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Hydrogen is an exception, as it will usually lose its electron. 93 rows this table shows the most common charges for atoms of the chemical elements. When a lead atom loses two electrons it exhibits a +2 charge. The oxidation. Do Lead Lose Electrons.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Do Lead Lose Electrons Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The electrons of the valence shell have less attraction to the nucleus and, as. Do Lead Lose Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Do Lead Lose Electrons Why does it gain electrons when losing them would be. In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). So, these. Do Lead Lose Electrons.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Do Lead Lose Electrons When a lead atom loses two electrons it exhibits a +2 charge. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The electrons. Do Lead Lose Electrons.
From www.youtube.com
Which elements tend to lose electrons? What charge will they Do Lead Lose Electrons The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. Hydrogen is an exception, as it will usually lose its electron. However, elements with a small atomic radius. Do Lead Lose Electrons.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID6788469 Do Lead Lose Electrons During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Why does it gain electrons when losing them would be. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. When a lead atom loses two electrons it exhibits a +2 charge. In. Do Lead Lose Electrons.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Do Lead Lose Electrons So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Hydrogen is an exception, as it will usually lose its electron. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). In general, metals will lose electrons to become. Do Lead Lose Electrons.
From www.slideserve.com
PPT Oxidation an atom loses one or more electrons . PowerPoint Do Lead Lose Electrons So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). This causes an increase in metallic character. Atoms tend to lose, gain, or share some valance electrons, making bonds. Do Lead Lose Electrons.
From www.slideserve.com
PPT Metals, Nonmetals, and Movement of Electrons PowerPoint Do Lead Lose Electrons In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. Why does it gain electrons when. Do Lead Lose Electrons.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Do Lead Lose Electrons In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. However, elements with a small atomic radius (such as fluorine) will have such a tight. So, these two electrons will be lost from the last shell, which means electrons will be lost. Do Lead Lose Electrons.
From www.slideserve.com
PPT CHEMICAL FORMULAS AND BONDING PowerPoint Presentation, free Do Lead Lose Electrons The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Elements that haven't got a very tight grip on these. Do Lead Lose Electrons.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Do Lead Lose Electrons When a lead atom loses two electrons it exhibits a +2 charge. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. You can use this table to predict whether an atom can. Do Lead Lose Electrons.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID5718047 Do Lead Lose Electrons This causes an increase in metallic character. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Why does it gain electrons when losing them would be. You can use this table to predict whether an atom can bond with another. Do Lead Lose Electrons.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Do Lead Lose Electrons This causes an increase in metallic character. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Elements that haven't got a very tight grip on these valence. Do Lead Lose Electrons.
From www.sciencephoto.com
Lead, atomic structure Stock Image C045/6429 Science Photo Library Do Lead Lose Electrons Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. During bond formation, the lead atom donates two electrons. Do Lead Lose Electrons.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Do Lead Lose Electrons So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Why does it gain electrons when losing them would be. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds.. Do Lead Lose Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Do Lead Lose Electrons However, elements with a small atomic radius (such as fluorine) will have such a tight. When a lead atom loses two electrons it exhibits a +2 charge. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Why does it gain. Do Lead Lose Electrons.
From periodictable.me
Lead Valence Electrons Lead Valency (Pb) with Dot Diagram Do Lead Lose Electrons In lead(ii) bromide electrolysis, lead is attracted to the cathode and gains electrons. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. Elements that haven't got a very tight grip on these valence electrons, will lose their valence electrons. Atoms tend to lose, gain, or share some valance. Do Lead Lose Electrons.
From www.slideserve.com
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download Do Lead Lose Electrons This causes an increase in metallic character. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Why does it gain. Do Lead Lose Electrons.