Fda Codes For Medical Devices . Classification product codes are a method of internally classifying and tracking medical devices. Cdrh and a subset of cber. Either class i, ii or iii, depending on its risk,. Enter a word from the product name or a keyword associated with the product name. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Exemptions from federal preemption of state and local medical device requirements: The name and product code identify the generic category of a device. This is the quickest way to find a product code. The product code assigned to a device is based upon the medical. This database contains device names and their associated product codes. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. The name and product code identify the generic category of a device for fda.
from smartdataweek.com
Enter a word from the product name or a keyword associated with the product name. Exemptions from federal preemption of state and local medical device requirements: Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. This is the quickest way to find a product code. Cdrh and a subset of cber. The product code assigned to a device is based upon the medical. Classification product codes are a method of internally classifying and tracking medical devices. Either class i, ii or iii, depending on its risk,. This database contains device names and their associated product codes.
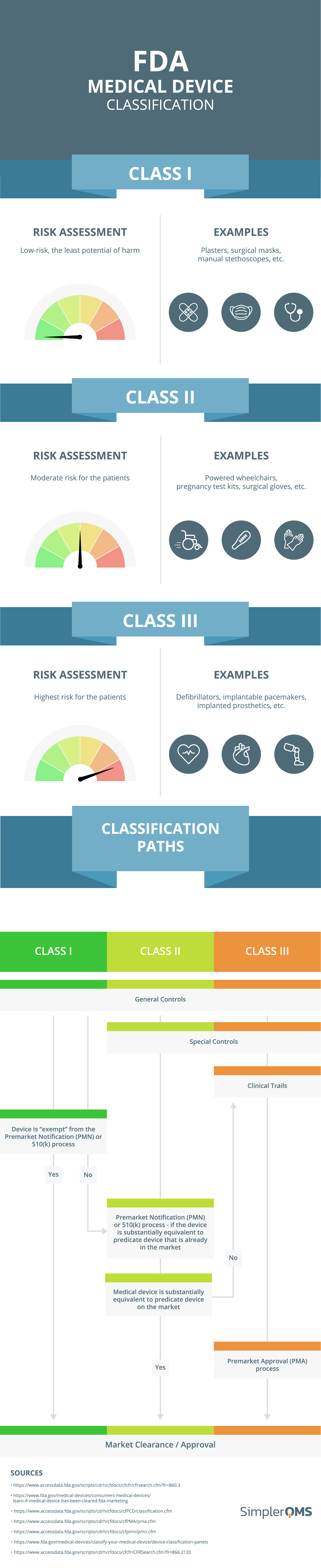
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024)
Fda Codes For Medical Devices A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. Cdrh and a subset of cber. The name and product code identify the generic category of a device. Classification product codes are a method of internally classifying and tracking medical devices. The product code assigned to a device is based upon the medical. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The name and product code identify the generic category of a device for fda. This database contains device names and their associated product codes. This is the quickest way to find a product code. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. Either class i, ii or iii, depending on its risk,. Enter a word from the product name or a keyword associated with the product name. Exemptions from federal preemption of state and local medical device requirements:
From www.regdesk.co
FDA Exemption for Class II Medical Devices RegDesk Fda Codes For Medical Devices Cdrh and a subset of cber. Either class i, ii or iii, depending on its risk,. This database contains device names and their associated product codes. Classification product codes are a method of internally classifying and tracking medical devices. The name and product code identify the generic category of a device. A list of all medical devices with their associated. Fda Codes For Medical Devices.
From klanjwrju.blob.core.windows.net
Fda Medical Device Labeling Guidance at Michael Crawford blog Fda Codes For Medical Devices The name and product code identify the generic category of a device. Cdrh and a subset of cber. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Classification product codes are a method of internally classifying and tracking medical devices. A list of all medical devices with their associated. Fda Codes For Medical Devices.
From www.orielstat.com
Understanding the FDA 510(k) Approval Process for Medical Devices Fda Codes For Medical Devices Exemptions from federal preemption of state and local medical device requirements: Either class i, ii or iii, depending on its risk,. Classification product codes are a method of internally classifying and tracking medical devices. The product code assigned to a device is based upon the medical. This database contains device names and their associated product codes. Enter a word from. Fda Codes For Medical Devices.
From www.linkedin.com
FDA Regulatory Pathways for Medical Devices Fda Codes For Medical Devices A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Enter a word from the product name or a keyword associated with the product name. The name and product code identify the. Fda Codes For Medical Devices.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Fda Codes For Medical Devices The product code assigned to a device is based upon the medical. Either class i, ii or iii, depending on its risk,. Cdrh and a subset of cber. The name and product code identify the generic category of a device for fda. The name and product code identify the generic category of a device. This is the quickest way to. Fda Codes For Medical Devices.
From hackaday.io
FDA Product Code, 510(k), Medical Device Standards/Regulations Fda Codes For Medical Devices This is the quickest way to find a product code. The name and product code identify the generic category of a device for fda. Classification product codes are a method of internally classifying and tracking medical devices. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: This database contains. Fda Codes For Medical Devices.
From www.slideserve.com
PPT FDA Code of Federal Regulations relating to medical devices Fda Codes For Medical Devices The product code assigned to a device is based upon the medical. Exemptions from federal preemption of state and local medical device requirements: Enter a word from the product name or a keyword associated with the product name. This database contains device names and their associated product codes. The name and product code identify the generic category of a device.. Fda Codes For Medical Devices.
From rfxcel.com
FDA National Drug Code Proposed Format Changes & Industry Impact Fda Codes For Medical Devices A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. This is the quickest way to find a product code. Classification product codes are a method of internally classifying and tracking medical devices. The product code assigned to a device is based upon the medical. This database contains device names and their. Fda Codes For Medical Devices.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Fda Codes For Medical Devices Either class i, ii or iii, depending on its risk,. Cdrh and a subset of cber. Exemptions from federal preemption of state and local medical device requirements: Classification product codes are a method of internally classifying and tracking medical devices. This database contains device names and their associated product codes. Any medical device approved by the fda center for devices. Fda Codes For Medical Devices.
From smartdataweek.com
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024) Fda Codes For Medical Devices This is the quickest way to find a product code. Exemptions from federal preemption of state and local medical device requirements: A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. Classification product codes are a method of internally classifying and tracking medical devices. The name and product code identify the generic. Fda Codes For Medical Devices.
From www.slideserve.com
PPT FDA Code of Federal Regulations relating to medical devices Fda Codes For Medical Devices Exemptions from federal preemption of state and local medical device requirements: Enter a word from the product name or a keyword associated with the product name. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The name and product code identify the generic category of a device. Classification product. Fda Codes For Medical Devices.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Fda Codes For Medical Devices Exemptions from federal preemption of state and local medical device requirements: This is the quickest way to find a product code. The name and product code identify the generic category of a device for fda. The name and product code identify the generic category of a device. A list of all medical devices with their associated classifications, product codes, fda. Fda Codes For Medical Devices.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Fda Codes For Medical Devices This database contains device names and their associated product codes. The name and product code identify the generic category of a device for fda. Classification product codes are a method of internally classifying and tracking medical devices. Cdrh and a subset of cber. Any medical device approved by the fda center for devices and radiological health is classified into one. Fda Codes For Medical Devices.
From www.slideserve.com
PPT FDA Code of Federal Regulations relating to medical devices Fda Codes For Medical Devices This database contains device names and their associated product codes. Cdrh and a subset of cber. The product code assigned to a device is based upon the medical. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. Classification product codes are a method of internally classifying and tracking medical devices. Any. Fda Codes For Medical Devices.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Codes For Medical Devices This database contains device names and their associated product codes. The product code assigned to a device is based upon the medical. This is the quickest way to find a product code. Either class i, ii or iii, depending on its risk,. Cdrh and a subset of cber. The name and product code identify the generic category of a device. Fda Codes For Medical Devices.
From mungfali.com
FDA Medical Device Label Symbols Fda Codes For Medical Devices The product code assigned to a device is based upon the medical. The name and product code identify the generic category of a device for fda. Classification product codes are a method of internally classifying and tracking medical devices. Exemptions from federal preemption of state and local medical device requirements: This is the quickest way to find a product code.. Fda Codes For Medical Devices.
From www.hardianhealth.com
A guide to FDA Diagnostic Radiology Product Codes — Hardian Health Fda Codes For Medical Devices A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. This is the quickest way to find a product code. Exemptions from federal preemption of state and local medical device requirements: Cdrh and a subset of cber. Enter a word from the product name or a keyword associated with the product name.. Fda Codes For Medical Devices.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Codes For Medical Devices This database contains device names and their associated product codes. Exemptions from federal preemption of state and local medical device requirements: Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Enter a word from the product name or a keyword associated with the product name. This is the quickest. Fda Codes For Medical Devices.
From vem-medical.com
Medical Device Manufacturing Fda Codes For Medical Devices A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. The name and product code identify the generic category of a device for fda. Either class i, ii or iii, depending on its risk,. Exemptions from federal preemption of state and local medical device requirements: The name and product code identify the. Fda Codes For Medical Devices.
From www.greenlight.guru
Ultimate Guide to 21 CFR Part 820 — FDA's Quality System Regulation Fda Codes For Medical Devices This database contains device names and their associated product codes. Cdrh and a subset of cber. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. This is the quickest way to find a product code. The name and product code identify the generic category of a device. The product code assigned. Fda Codes For Medical Devices.
From www.thinqbetter.com
FDA approval for medical devices FDA 510k and more. Fda Codes For Medical Devices Either class i, ii or iii, depending on its risk,. Cdrh and a subset of cber. The name and product code identify the generic category of a device for fda. This database contains device names and their associated product codes. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes:. Fda Codes For Medical Devices.
From www.hardianhealth.com
A guide to FDA Diagnostic Radiology Product Codes — Hardian Health Fda Codes For Medical Devices Exemptions from federal preemption of state and local medical device requirements: A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. The name and product code identify the generic category of a device for fda. The name and product code identify the generic category of a device. Any medical device approved by. Fda Codes For Medical Devices.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Fda Codes For Medical Devices This is the quickest way to find a product code. The name and product code identify the generic category of a device. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. This database contains device names and their associated product codes. Enter a word from the product name or a keyword. Fda Codes For Medical Devices.
From innolitics.com
2013 FDA Guidance Medical Device Classification Product Codes Fda Codes For Medical Devices Enter a word from the product name or a keyword associated with the product name. Exemptions from federal preemption of state and local medical device requirements: Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The name and product code identify the generic category of a device. Either class. Fda Codes For Medical Devices.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Codes For Medical Devices The name and product code identify the generic category of a device for fda. Classification product codes are a method of internally classifying and tracking medical devices. This is the quickest way to find a product code. This database contains device names and their associated product codes. A list of all medical devices with their associated classifications, product codes, fda. Fda Codes For Medical Devices.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Fda Codes For Medical Devices Classification product codes are a method of internally classifying and tracking medical devices. Exemptions from federal preemption of state and local medical device requirements: This database contains device names and their associated product codes. This is the quickest way to find a product code. The name and product code identify the generic category of a device for fda. The name. Fda Codes For Medical Devices.
From ramtechno.com
What Are the Three FDA Classes for Medical Devices? RAM Technologies Fda Codes For Medical Devices Exemptions from federal preemption of state and local medical device requirements: This database contains device names and their associated product codes. Enter a word from the product name or a keyword associated with the product name. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. The product code assigned to a. Fda Codes For Medical Devices.
From mavink.com
Fda Medical Device Classification Chart Fda Codes For Medical Devices Classification product codes are a method of internally classifying and tracking medical devices. Exemptions from federal preemption of state and local medical device requirements: A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. This database contains device names and their associated product codes. This is the quickest way to find a. Fda Codes For Medical Devices.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Codes For Medical Devices Cdrh and a subset of cber. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The name and product code identify the generic category of a device. Either class i, ii. Fda Codes For Medical Devices.
From www.slideserve.com
PPT FDA Code of Federal Regulations relating to medical devices Fda Codes For Medical Devices This database contains device names and their associated product codes. The product code assigned to a device is based upon the medical. Cdrh and a subset of cber. A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. The name and product code identify the generic category of a device. Exemptions from. Fda Codes For Medical Devices.
From www.nicelabel.com
FDA UDI compliant labelling NiceLabel Fda Codes For Medical Devices A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. The name and product code identify the generic category of a device. Exemptions from federal preemption of state and local medical device requirements: Cdrh and a subset of cber. Any medical device approved by the fda center for devices and radiological health. Fda Codes For Medical Devices.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Fda Codes For Medical Devices Enter a word from the product name or a keyword associated with the product name. Either class i, ii or iii, depending on its risk,. The name and product code identify the generic category of a device for fda. Exemptions from federal preemption of state and local medical device requirements: The product code assigned to a device is based upon. Fda Codes For Medical Devices.
From www.greenlight.guru
Medical Device Classifications Determine Your Device Class Fda Codes For Medical Devices This is the quickest way to find a product code. Exemptions from federal preemption of state and local medical device requirements: This database contains device names and their associated product codes. The name and product code identify the generic category of a device for fda. The product code assigned to a device is based upon the medical. The name and. Fda Codes For Medical Devices.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Fda Codes For Medical Devices This database contains device names and their associated product codes. Exemptions from federal preemption of state and local medical device requirements: A list of all medical devices with their associated classifications, product codes, fda premarket review organizations, and other. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The. Fda Codes For Medical Devices.
From www.reedtech.com
Unique Device Identification (UDI) Lexis Nexis Reed Tech Fda Codes For Medical Devices Either class i, ii or iii, depending on its risk,. Enter a word from the product name or a keyword associated with the product name. The name and product code identify the generic category of a device for fda. Cdrh and a subset of cber. The name and product code identify the generic category of a device. The product code. Fda Codes For Medical Devices.