Chlorine Atoms Diatomic Molecule . There are seven diatomic elements: Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. It is also the strongest of the chemical bonds. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. the chemical formula of a homonuclear diatomic molecule can be determined using a modified version of the rules presented. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. It's possible that an eighth element forms a diatomic molecule. These elements can exist in pure form in other arrangements. this is because only five elements form stable diatomic molecules at standard temperature and pressure: Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. A covalent bond occurs when electron pairs are shared between two atoms; Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures.
from www.alamy.com
Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. this is because only five elements form stable diatomic molecules at standard temperature and pressure: consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. It is also the strongest of the chemical bonds. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. These elements can exist in pure form in other arrangements.
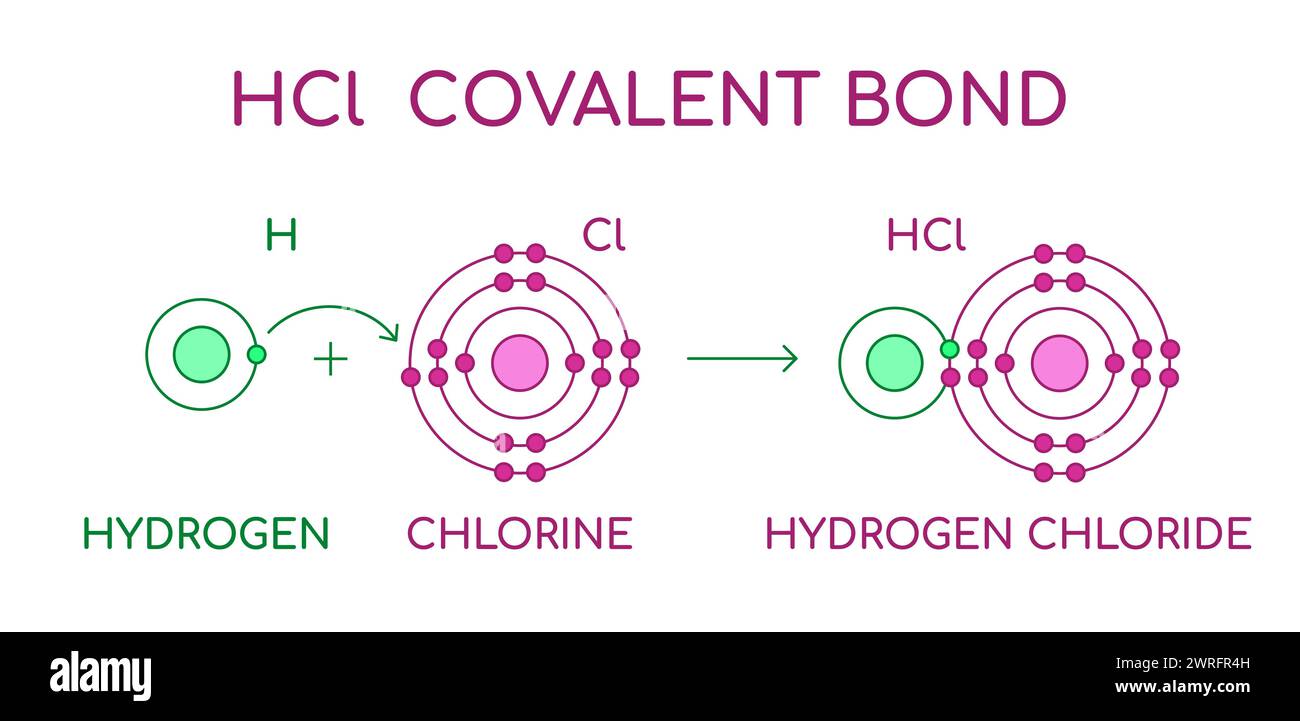
HCl Hydrogen Chloride covalent bond. Diatomic molecule, consisting of a
Chlorine Atoms Diatomic Molecule other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. this is because only five elements form stable diatomic molecules at standard temperature and pressure: The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. A covalent bond occurs when electron pairs are shared between two atoms; There are seven diatomic elements: It's possible that an eighth element forms a diatomic molecule. It is also the strongest of the chemical bonds. the chemical formula of a homonuclear diatomic molecule can be determined using a modified version of the rules presented. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. These elements can exist in pure form in other arrangements. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine.
From www.alamy.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Atoms Diatomic Molecule These elements can exist in pure form in other arrangements. the chemical formula of a homonuclear diatomic molecule can be determined using a modified version of the rules presented. A covalent bond occurs when electron pairs are shared between two atoms; It's possible that an eighth element forms a diatomic molecule. It is also the strongest of the chemical. Chlorine Atoms Diatomic Molecule.
From www.istockphoto.com
Chemistry Model Salt Molecule Diatomic Sodium Chlorine Nacl Scientific Chlorine Atoms Diatomic Molecule Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. There are seven diatomic elements: The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. this is because only five elements form stable diatomic molecules at standard temperature and pressure: diatomic elements are pure elements that form molecules consisting of two atoms bonded together. the chemical formula of a homonuclear diatomic. Chlorine Atoms Diatomic Molecule.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Atoms Diatomic Molecule chlorine contains 7 electrons in its outer shell and needs one to complete its octet. Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. There are seven diatomic elements: It is also the strongest of the chemical bonds. These elements can exist in pure form in other arrangements. The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. . Chlorine Atoms Diatomic Molecule.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Atoms Diatomic Molecule It's possible that an eighth element forms a diatomic molecule. A covalent bond occurs when electron pairs are shared between two atoms; It is also the strongest of the chemical bonds. There are seven diatomic elements: The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of. Chlorine Atoms Diatomic Molecule.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Atoms Diatomic Molecule chlorine contains 7 electrons in its outer shell and needs one to complete its octet. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Using the molecular orbital approach to describe the bonding in hcl,. Chlorine Atoms Diatomic Molecule.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure Chlorine Atoms Diatomic Molecule this is because only five elements form stable diatomic molecules at standard temperature and pressure: consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. There are seven diatomic elements: Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. diatomic elements are pure elements that. Chlorine Atoms Diatomic Molecule.
From www.scienceabc.com
Diatomic Molecules Definition, Explanation And Examples Chlorine Atoms Diatomic Molecule Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. other elements also exist naturally as diatomic molecules —a molecule with only two ato. Chlorine Atoms Diatomic Molecule.
From www.chemistrylearner.com
Diatomic Molecules Definition and List Chlorine Atoms Diatomic Molecule Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. It is also the strongest of the chemical bonds. diatomic elements are pure elements that form molecules consisting of two atoms bonded. Chlorine Atoms Diatomic Molecule.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Atoms Diatomic Molecule A covalent bond occurs when electron pairs are shared between two atoms; Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. It is also the. Chlorine Atoms Diatomic Molecule.
From www.alamy.com
HCl Hydrogen Chloride covalent bond. Diatomic molecule, consisting of a Chlorine Atoms Diatomic Molecule this is because only five elements form stable diatomic molecules at standard temperature and pressure: Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. These elements can exist. Chlorine Atoms Diatomic Molecule.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Photo Chlorine Atoms Diatomic Molecule It is also the strongest of the chemical bonds. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. These elements can exist in pure form in other arrangements. A covalent bond occurs when electron pairs are shared between two atoms; Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. this is. Chlorine Atoms Diatomic Molecule.
From www.shutterstock.com
404 Diatomic Molecule Images, Stock Photos & Vectors Shutterstock Chlorine Atoms Diatomic Molecule the chemical formula of a homonuclear diatomic molecule can be determined using a modified version of the rules presented. It's possible that an eighth element forms a diatomic molecule. consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. A. Chlorine Atoms Diatomic Molecule.
From www.vectorstock.com
Cl2 chlorine molecule Royalty Free Vector Image Chlorine Atoms Diatomic Molecule Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. other elements also exist naturally. Chlorine Atoms Diatomic Molecule.
From www.scienceabc.com
Diatomic Molecules Definition, Explanation And Examples Chlorine Atoms Diatomic Molecule There are seven diatomic elements: Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. this is because only five elements form stable diatomic. Chlorine Atoms Diatomic Molecule.
From sciencenotes.org
What Are the 7 Diatomic Elements? Definition and List Chlorine Atoms Diatomic Molecule There are seven diatomic elements: Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. the chemical formula of a homonuclear diatomic. Chlorine Atoms Diatomic Molecule.
From www.istockphoto.com
Chlorine Molecular Structure Isolated On White Stock Photo Download Chlorine Atoms Diatomic Molecule this is because only five elements form stable diatomic molecules at standard temperature and pressure: chlorine contains 7 electrons in its outer shell and needs one to complete its octet. It is also the strongest of the chemical bonds. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. These elements can exist in pure form in other arrangements. The. Chlorine Atoms Diatomic Molecule.
From www.alamy.com
Chemistry model molecule diatomic chlorine CL2 scientific element Chlorine Atoms Diatomic Molecule Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. other elements also exist naturally. Chlorine Atoms Diatomic Molecule.
From www.istockphoto.com
Chemistry Model Molecule Diatomic Chlorine Cl2 Scientific Element Chlorine Atoms Diatomic Molecule A covalent bond occurs when electron pairs are shared between two atoms; Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. There are seven diatomic elements: consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. It is also the strongest of the chemical bonds. Hydrogen, nitrogen,. Chlorine Atoms Diatomic Molecule.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Atoms Diatomic Molecule It is also the strongest of the chemical bonds. These elements can exist in pure form in other arrangements. There are seven diatomic elements: consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. Sodium has 1 electron in its outer shell, which it donates to chlorine to form. Chlorine Atoms Diatomic Molecule.
From fineartamerica.com
Bond Formation In Chlorine Molecule Photograph by Chlorine Atoms Diatomic Molecule Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. It's possible that an eighth element forms a diatomic molecule. There are seven diatomic elements: . Chlorine Atoms Diatomic Molecule.
From brainly.com
The diatomic molecule of chlorine, cl2, is held together by a(n Chlorine Atoms Diatomic Molecule Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. the chemical formula of a homonuclear diatomic molecule can be determined using a modified. Chlorine Atoms Diatomic Molecule.
From www.scienceabc.com
Diatomic Molecules Definition, Explanation And Examples Chlorine Atoms Diatomic Molecule the chemical formula of a homonuclear diatomic molecule can be determined using a modified version of the rules presented. These elements can exist in pure form in other arrangements. Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to. Chlorine Atoms Diatomic Molecule.
From mammothmemory.net
Diatomic molecules have two of the same element Chlorine Atoms Diatomic Molecule diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms. Chlorine Atoms Diatomic Molecule.
From www.vecteezy.com
Chemistry model molecule diatomic chlorine CL2 scientific element Chlorine Atoms Diatomic Molecule There are seven diatomic elements: this is because only five elements form stable diatomic molecules at standard temperature and pressure: These elements can exist in pure form in other arrangements. consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. diatomic elements are pure elements that form. Chlorine Atoms Diatomic Molecule.
From stock.adobe.com
Chemistry model molecule diatomic chlorine CL2 scientific element Chlorine Atoms Diatomic Molecule Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. These elements can exist in pure. Chlorine Atoms Diatomic Molecule.
From www.istockphoto.com
Chlorine Molecular Structure Isolated On Black Stock Photo Download Chlorine Atoms Diatomic Molecule chlorine contains 7 electrons in its outer shell and needs one to complete its octet. It's possible that an eighth element forms a diatomic molecule. Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals. Chlorine Atoms Diatomic Molecule.
From www.sciencephoto.com
Chlorine molecules, illustration Stock Image C055/3835 Science Chlorine Atoms Diatomic Molecule consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. A covalent bond occurs when electron pairs are shared between two atoms; The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. this is because only five elements form. Chlorine Atoms Diatomic Molecule.
From www.kindpng.com
Sensors Chlorine Info Support Homonuclear Diatomic Molecule Hcl, HD Chlorine Atoms Diatomic Molecule These elements can exist in pure form in other arrangements. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. consider, for example, the hcl molecule, whose lewis electron structure has three lone pairs of electrons on the chlorine atom. There are seven diatomic elements: It's possible that an eighth element forms a diatomic. Chlorine Atoms Diatomic Molecule.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl2 Diatomic Chlorine YouTube Chlorine Atoms Diatomic Molecule Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. the chemical formula of a homonuclear diatomic molecule can. Chlorine Atoms Diatomic Molecule.
From www.istockphoto.com
Chlorine Molecular Structure Isolated On White Stock Photo Download Chlorine Atoms Diatomic Molecule Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. the chemical formula of a homonuclear diatomic molecule can be determined using a modified version of the rules presented. this is because. Chlorine Atoms Diatomic Molecule.
From www.alamy.com
Chemistry model salt molecule diatomic sodium chlorine NaCl scientific Chlorine Atoms Diatomic Molecule There are seven diatomic elements: The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. A covalent bond occurs when electron pairs are shared between two atoms; Using the molecular orbital approach to describe the bonding in hcl, we can see from figure. Chlorine Atoms Diatomic Molecule.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Atoms Diatomic Molecule Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. chlorine contains 7 electrons in its outer shell and needs one to complete its octet. It is also the strongest of the chemical. Chlorine Atoms Diatomic Molecule.
From ar.inspiredpencil.com
Chlorine Molecule Structure Chlorine Atoms Diatomic Molecule Using the molecular orbital approach to describe the bonding in hcl, we can see from figure \(\pageindex{10}\) that the 1 s orbital of atomic hydrogen is closest in energy to the 3 p orbitals of chlorine. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. There are seven diatomic elements: These elements can exist. Chlorine Atoms Diatomic Molecule.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Atoms Diatomic Molecule It's possible that an eighth element forms a diatomic molecule. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. this is because only five elements form stable diatomic molecules at standard temperature and pressure: other elements also exist naturally as diatomic molecules —a molecule with only two ato ms (table 5.2.1 5.2. chlorine contains 7 electrons in its. Chlorine Atoms Diatomic Molecule.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Chlorine Atoms Diatomic Molecule this is because only five elements form stable diatomic molecules at standard temperature and pressure: the chemical formula of a homonuclear diatomic molecule can be determined using a modified version of the rules presented. Bromine and iodine form homonuclear diatomic molecules at slightly higher temperatures. The gases hydrogen, nitrogen, oxygen, fluorine, and chlorine. These elements can exist in. Chlorine Atoms Diatomic Molecule.