Standard Enthalpy Of Formation For Nh3 . Chem., 1968, 72, 2, 742,. It is a thermodynamic measure that represents the system's overall heat content. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; It is equivalent to the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction:
from www.youtube.com
the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: It is equivalent to the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Chem., 1968, 72, 2, 742,. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; It is a thermodynamic measure that represents the system's overall heat content.
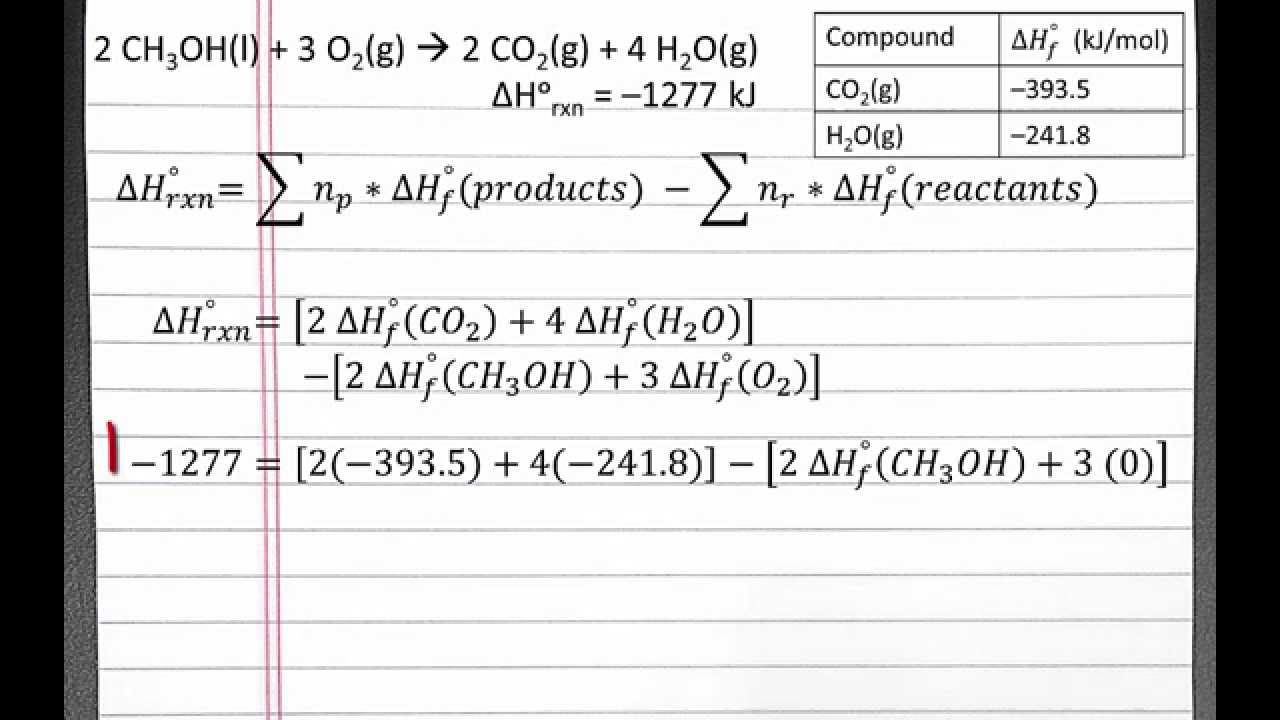
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies
Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. It is a thermodynamic measure that represents the system's overall heat content. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. It is equivalent to the. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Chem., 1968, 72, 2, 742,.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJmol. If the Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. It is equivalent to the. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; 136. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol^1 . If the Standard Enthalpy Of Formation For Nh3 It is equivalent to the. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. definition and explanation of the terms standard state and standard enthalpy of formation, with. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The enthalpy of formation of ammonia is −46.0 kJ mol−1. The enthalpy Standard Enthalpy Of Formation For Nh3 It is equivalent to the. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation For Nh3.
From www.researchgate.net
(a) Calculated formation enthalpy of various NH 3 −H 2 compounds with Standard Enthalpy Of Formation For Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. It is equivalent to the. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. Chem., 1968, 72, 2, 742,. 136 rows standard enthalpy change of. Standard Enthalpy Of Formation For Nh3.
From hxesyzijm.blob.core.windows.net
Standard Enthalpy Of Formation Database at Kevin Leblanc blog Standard Enthalpy Of Formation For Nh3 Chem., 1968, 72, 2, 742,. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this table lists the standard enthalpies (δh°), the free energies (δg°). Standard Enthalpy Of Formation For Nh3.
From www.chegg.com
Solved The standard enthalpy of formation for NH3(g) is Standard Enthalpy Of Formation For Nh3 Chem., 1968, 72, 2, 742,. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: It is a thermodynamic measure that represents the system's overall heat content. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; . Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol ^1 . If the Standard Enthalpy Of Formation For Nh3 \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; It is equivalent to the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. definition and explanation of the terms standard state and standard enthalpy of formation,. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol^1 . If the Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: definition and explanation of the. Standard Enthalpy Of Formation For Nh3.
From zavierfersprince.blogspot.com
Ammonia Nh3 Reacts With Hydrogen Chloride to Form Ammonium Chloride Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Chem., 1968, 72, 2, 742,. It is equivalent to. Standard Enthalpy Of Formation For Nh3.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation For Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Chem., 1968, 72, 2, 742,. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. definition and explanation of the terms standard state and standard enthalpy. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
Enthalpy of formation of NH3 is X kJ and HH H, HN H are Standard Enthalpy Of Formation For Nh3 this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. It is equivalent to the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. definition and explanation of the terms standard state and standard enthalpy of formation, with. Standard Enthalpy Of Formation For Nh3.
From kunduz.com
[ANSWERED] 1 The standard enthalpy of formation of NH3 is 46 0 kJ mol Standard Enthalpy Of Formation For Nh3 this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. It is a thermodynamic measure that represents the system's overall heat content. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. \[ 6c\left (s, graphite \right. Standard Enthalpy Of Formation For Nh3.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation For Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: It is equivalent to the. this table lists the standard enthalpies (δh°), the free energies. Standard Enthalpy Of Formation For Nh3.
From www.youtube.com
The standard enthalpy of formation of NH3 is 46 kJ/ mol Heat of Standard Enthalpy Of Formation For Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. Standard Enthalpy Of Formation For Nh3.
From www.numerade.com
SOLVED According to the information above what is the standard Standard Enthalpy Of Formation For Nh3 It is equivalent to the. Chem., 1968, 72, 2, 742,. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. definition and explanation of the terms standard state and. Standard Enthalpy Of Formation For Nh3.
From edurev.in
The standard enthalpy of formation of Nh3 is 46.0 kJ per mol if the Standard Enthalpy Of Formation For Nh3 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. It is a thermodynamic measure that represents the system's overall heat content. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. this table lists the standard enthalpies (δh°), the. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol^1 . If the Standard Enthalpy Of Formation For Nh3 \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. It is a thermodynamic measure that represents the system's overall heat content. the standard enthalpy of formation of. Standard Enthalpy Of Formation For Nh3.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation For Nh3 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. It is equivalent to the. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; Chem., 1968, 72, 2, 742,. the standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation For Nh3.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation For Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation For Nh3.
From www.beinyu.com
Calculating Enthalpy Of Ammonia Formation Standard Enthalpy Of Formation For Nh3 this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Chem., 1968, 72, 2, 742,. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJmo17. If the Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. It is equivalent to the. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy. Standard Enthalpy Of Formation For Nh3.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation For Nh3 Chem., 1968, 72, 2, 742,. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation For Nh3.
From priaxon.com
What Is Standard Enthalpy Of Formation Of Nh3 Gas Templates Printable Standard Enthalpy Of Formation For Nh3 \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; It is a thermodynamic measure that represents the system's overall heat content. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 136 rows standard enthalpy change of. Standard Enthalpy Of Formation For Nh3.
From www.numerade.com
SOLVEDThe standard enthalpy for the gasphase of the Standard Enthalpy Of Formation For Nh3 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; Chem., 1968, 72, 2, 742,. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation. Standard Enthalpy Of Formation For Nh3.
From askfilo.com
What is the standard enthalpy of formation of NH3 gas, standard heat of Standard Enthalpy Of Formation For Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. It is a thermodynamic measure that represents the system's overall heat content. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: this table lists. Standard Enthalpy Of Formation For Nh3.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard Standard Enthalpy Of Formation For Nh3 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. It is equivalent to the. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. definition and explanation of the terms standard state and standard enthalpy of formation, with. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Chem., 1968, 72, 2, 742,. It is equivalent to. Standard Enthalpy Of Formation For Nh3.
From edurev.in
The standard enthalpy of for mation of NH3 is andndash; 46.0 kJ Standard Enthalpy Of Formation For Nh3 Chem., 1968, 72, 2, 742,. It is equivalent to the. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. \[ 6c\left (s, graphite \right ) + 6h_{2}\left. Standard Enthalpy Of Formation For Nh3.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpy Of Formation For Nh3 It is equivalent to the. Chem., 1968, 72, 2, 742,. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. It is a thermodynamic measure that represents the system's. Standard Enthalpy Of Formation For Nh3.
From www.numerade.com
SOLVEDThe standard enthalpy of formation of NH3 is 46.0 kJ mol^1. If Standard Enthalpy Of Formation For Nh3 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left. Standard Enthalpy Of Formation For Nh3.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; this table lists. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol^1 . If the Standard Enthalpy Of Formation For Nh3 \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; It is equivalent to the. Chem., 1968, 72, 2, 742,. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: definition and explanation of the terms standard. Standard Enthalpy Of Formation For Nh3.
From www.numerade.com
SOLVEDUse the following standard enthalpies of formation to estimate Standard Enthalpy Of Formation For Nh3 It is a thermodynamic measure that represents the system's overall heat content. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Chem., 1968, 72, 2, 742,.. Standard Enthalpy Of Formation For Nh3.
From www.numerade.com
SOLVED The standard enthalpy of formation of NH3(g) at 298 K is 46 kJ Standard Enthalpy Of Formation For Nh3 this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; the standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation For Nh3.
From www.toppr.com
The standard enthalpy of formation of NH3 is 46.0 kJ mol^1 . If the Standard Enthalpy Of Formation For Nh3 It is equivalent to the. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: 136 rows standard enthalpy change of formation (data table) these tables include. Standard Enthalpy Of Formation For Nh3.