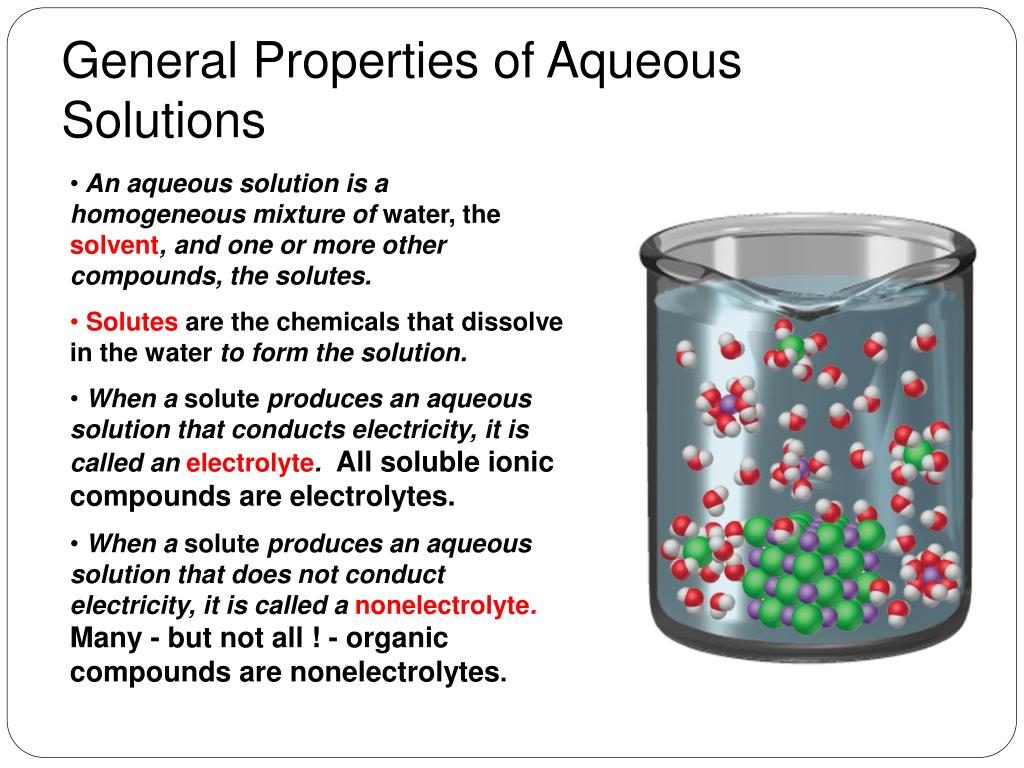
How To Create An Aqueous Solution . Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: Define a solution and describe the parts of a solution. The solutes are dissolved molecules and ions that are surrounded by water molecules. The solvent in aqueous solutions is water, which. Describe how an aqueous solution is formed from both ionic compounds. Decide on the solution's concentration. There are seven steps to making a solution. The concentration describes how much solute is in. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. Calculate molarity of a solution. To understand how and why solutions form. An aqueous solution is a chemical solution in which the solvent is water. You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water).
from www.slideserve.com
An aqueous solution is a chemical solution in which the solvent is water. The concentration describes how much solute is in. Calculate molarity of a solution. The solvent in aqueous solutions is water, which. Define a solution and describe the parts of a solution. You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). To understand how and why solutions form. Decide on the solution's concentration. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water.
PPT UNIT 5 PowerPoint Presentation, free download ID2276550
How To Create An Aqueous Solution Define a solution and describe the parts of a solution. An aqueous solution is a chemical solution in which the solvent is water. You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: The concentration describes how much solute is in. Calculate molarity of a solution. Define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds. The solvent in aqueous solutions is water, which. The solutes are dissolved molecules and ions that are surrounded by water molecules. To understand how and why solutions form. Decide on the solution's concentration. There are seven steps to making a solution. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo How To Create An Aqueous Solution The concentration describes how much solute is in. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: Define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds. The solutes are dissolved molecules and ions that are surrounded by water molecules.. How To Create An Aqueous Solution.
From www.teachoo.com
Why does aqueous solution of an acid conduct electricity? Class 10 How To Create An Aqueous Solution Describe how an aqueous solution is formed from both ionic compounds. You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). The solutes are dissolved molecules and ions that are surrounded by water molecules. The concentration describes how much solute is in. Define a solution and describe the parts. How To Create An Aqueous Solution.
From paperwriter.web.fc2.com
Reactions in aqueous solution experiment 20 metathesis How To Create An Aqueous Solution An aqueous solution is a chemical solution in which the solvent is water. The solutes are dissolved molecules and ions that are surrounded by water molecules. Decide on the solution's concentration. Define a solution and describe the parts of a solution. Calculate molarity of a solution. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water.. How To Create An Aqueous Solution.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples How To Create An Aqueous Solution Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. The concentration describes how much solute is in. There are seven steps to making a solution. The solvent in aqueous solutions is water, which. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: You will need a. How To Create An Aqueous Solution.
From www.chegg.com
Solved Consider an aqueous solution with a concentration of How To Create An Aqueous Solution There are seven steps to making a solution. The concentration describes how much solute is in. Describe how an aqueous solution is formed from both ionic compounds. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: An aqueous solution is a chemical solution in which the solvent is water. Define what. How To Create An Aqueous Solution.
From www.slideserve.com
PPT Ions in Aqueous Solution PowerPoint Presentation, free download How To Create An Aqueous Solution Describe how an aqueous solution is formed from both ionic compounds. Decide on the solution's concentration. There are seven steps to making a solution. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: The solutes are dissolved molecules and ions that are surrounded by water molecules. An aqueous solution is a. How To Create An Aqueous Solution.
From askanydifference.com
Liquid vs Aqueous Difference and Comparison How To Create An Aqueous Solution An aqueous solution is a chemical solution in which the solvent is water. The solvent in aqueous solutions is water, which. There are seven steps to making a solution. Define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds. The concentration describes how much solute is in. The solutes. How To Create An Aqueous Solution.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution How To Create An Aqueous Solution To understand how and why solutions form. Decide on the solution's concentration. Describe how an aqueous solution is formed from both ionic compounds. The solutes are dissolved molecules and ions that are surrounded by water molecules. There are seven steps to making a solution. An aqueous solution is a chemical solution in which the solvent is water. Define what makes. How To Create An Aqueous Solution.
From www.bigstockphoto.com
Ions Aqueous Solution Image & Photo (Free Trial) Bigstock How To Create An Aqueous Solution Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. Decide on the solution's concentration. To understand how and why solutions form. There are seven steps to making a solution. The solvent in aqueous solutions is water, which. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution:. How To Create An Aqueous Solution.
From www.slideserve.com
PPT Ch 4. Chemical Quantities and Aqueous Reactions PowerPoint How To Create An Aqueous Solution Define a solution and describe the parts of a solution. The solvent in aqueous solutions is water, which. There are seven steps to making a solution. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. Calculate molarity of a solution. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base). How To Create An Aqueous Solution.
From www.slideserve.com
PPT Aqueous PowerPoint Presentation, free download ID5171789 How To Create An Aqueous Solution The solutes are dissolved molecules and ions that are surrounded by water molecules. Decide on the solution's concentration. Define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds. To understand how and why solutions form. You will need a balance to weigh out the solute and a graduated cylinder. How To Create An Aqueous Solution.
From www.chegg.com
Solved Consider an aqueous solution with an H₂O How To Create An Aqueous Solution The concentration describes how much solute is in. Describe how an aqueous solution is formed from both ionic compounds. The solvent in aqueous solutions is water, which. An aqueous solution is a chemical solution in which the solvent is water. Define a solution and describe the parts of a solution. To understand how and why solutions form. You will need. How To Create An Aqueous Solution.
From www.pinterest.com.au
Aqueous Solution Chemistry experiments, Solutions, Chemistry study guide How To Create An Aqueous Solution Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: There are seven steps to making a solution. To understand how and why solutions form. The solvent in aqueous solutions is water, which. Decide on the solution's concentration. The concentration describes how much solute is in. Calculate molarity of a solution. Define. How To Create An Aqueous Solution.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free How To Create An Aqueous Solution The concentration describes how much solute is in. The solvent in aqueous solutions is water, which. Decide on the solution's concentration. Calculate molarity of a solution. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: There are seven steps to making a solution. Define a solution and describe the parts of. How To Create An Aqueous Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry How To Create An Aqueous Solution An aqueous solution is a chemical solution in which the solvent is water. The solvent in aqueous solutions is water, which. The concentration describes how much solute is in. Define a solution and describe the parts of a solution. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: Decide on the. How To Create An Aqueous Solution.
From www.numerade.com
SOLVED Please respond to the following question Match each name below How To Create An Aqueous Solution Calculate molarity of a solution. The concentration describes how much solute is in. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: There are seven steps to making a solution. The solvent in aqueous solutions is water, which. Describe how an aqueous solution is formed from both ionic compounds. Define a. How To Create An Aqueous Solution.
From studylib.net
Aqueoussolution reactions How To Create An Aqueous Solution An aqueous solution is a chemical solution in which the solvent is water. To understand how and why solutions form. You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). Decide on the solution's concentration. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong. How To Create An Aqueous Solution.
From ar.inspiredpencil.com
Aqueous Solution How To Create An Aqueous Solution Define a solution and describe the parts of a solution. You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). An aqueous solution is a chemical solution in which the solvent is water. The solutes are dissolved molecules and ions that are surrounded by water molecules. Describe how an. How To Create An Aqueous Solution.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 How To Create An Aqueous Solution To understand how and why solutions form. The solvent in aqueous solutions is water, which. There are seven steps to making a solution. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. The concentration describes how much. How To Create An Aqueous Solution.
From www.chegg.com
Solved Consider an aqueous solution with a concentration How To Create An Aqueous Solution The solvent in aqueous solutions is water, which. Define a solution and describe the parts of a solution. The concentration describes how much solute is in. An aqueous solution is a chemical solution in which the solvent is water. The solutes are dissolved molecules and ions that are surrounded by water molecules. Decide on the solution's concentration. There are seven. How To Create An Aqueous Solution.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry How To Create An Aqueous Solution Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). Describe how an aqueous solution is formed from both ionic compounds. The concentration describes how much solute is in. An aqueous. How To Create An Aqueous Solution.
From www.youtube.com
Aqueous Solution Chemistry YouTube How To Create An Aqueous Solution Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: The solvent in aqueous solutions is water, which. Describe how an aqueous solution is formed from both ionic compounds. Decide on the solution's concentration. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. Define a solution and. How To Create An Aqueous Solution.
From www.chegg.com
Solved Consider an aqueous solution with a concentration of How To Create An Aqueous Solution To understand how and why solutions form. Decide on the solution's concentration. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. The solutes are dissolved molecules and ions that are surrounded by water molecules. Calculate molarity of a solution. Define a solution and describe the parts of a solution. Sodium hydroxide is an ionic compound. How To Create An Aqueous Solution.
From emmanuel-well-rivera.blogspot.com
Why Does an Aqueous Solution of an Acid Conduct Electricity How To Create An Aqueous Solution There are seven steps to making a solution. Decide on the solution's concentration. To understand how and why solutions form. Describe how an aqueous solution is formed from both ionic compounds. Calculate molarity of a solution. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: The solutes are dissolved molecules and. How To Create An Aqueous Solution.
From chemwiki.ucdavis.edu
Page Not Found Chemwiki How To Create An Aqueous Solution Decide on the solution's concentration. The solutes are dissolved molecules and ions that are surrounded by water molecules. The concentration describes how much solute is in. An aqueous solution is a chemical solution in which the solvent is water. There are seven steps to making a solution. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in. How To Create An Aqueous Solution.
From www.transtutors.com
(Get Answer) Aqueous Solutions Of Sodium Bicarbonate And Sulfuric How To Create An Aqueous Solution The solutes are dissolved molecules and ions that are surrounded by water molecules. The concentration describes how much solute is in. Calculate molarity of a solution. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: The solvent in aqueous solutions is water, which. Define what makes an “aqueous solution.” explain the. How To Create An Aqueous Solution.
From www.slideserve.com
PPT Chemical Reactions in Aqueous Solutions PowerPoint Presentation How To Create An Aqueous Solution To understand how and why solutions form. Decide on the solution's concentration. An aqueous solution is a chemical solution in which the solvent is water. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. Describe how an aqueous solution is formed from both ionic compounds. Calculate molarity of a solution. Sodium hydroxide is an ionic. How To Create An Aqueous Solution.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution How To Create An Aqueous Solution Define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. The solvent in aqueous solutions is water, which. An aqueous solution is a chemical solution in which the solvent is water. To understand how and. How To Create An Aqueous Solution.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation How To Create An Aqueous Solution Describe how an aqueous solution is formed from both ionic compounds. The solvent in aqueous solutions is water, which. The concentration describes how much solute is in. Decide on the solution's concentration. To understand how and why solutions form. Define a solution and describe the parts of a solution. There are seven steps to making a solution. Define what makes. How To Create An Aqueous Solution.
From studylib.net
Reactions in Aqueous Solutions How To Create An Aqueous Solution The concentration describes how much solute is in. The solutes are dissolved molecules and ions that are surrounded by water molecules. Decide on the solution's concentration. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. Describe how an aqueous solution is formed from both ionic compounds. Calculate molarity of a solution. The solvent in aqueous. How To Create An Aqueous Solution.
From www.slideserve.com
PPT Reactions in Aqueous Solutions PowerPoint Presentation, free How To Create An Aqueous Solution You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). The concentration describes how much solute is in. To understand how and why solutions form. An aqueous solution is a chemical solution in which the solvent is water. The solutes are dissolved molecules and ions that are surrounded by. How To Create An Aqueous Solution.
From ar.inspiredpencil.com
Aqueous Solution How To Create An Aqueous Solution Decide on the solution's concentration. The solutes are dissolved molecules and ions that are surrounded by water molecules. The solvent in aqueous solutions is water, which. Describe how an aqueous solution is formed from both ionic compounds. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. To understand how and why solutions form. Define a. How To Create An Aqueous Solution.
From www.slideserve.com
PPT AQUEOUS SOLUTIONS PowerPoint Presentation, free download ID3930687 How To Create An Aqueous Solution An aqueous solution is a chemical solution in which the solvent is water. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base) in aqueous solution: The solutes are dissolved molecules and ions that are surrounded by water molecules. You will need a balance to weigh out the solute and a graduated cylinder to measure. How To Create An Aqueous Solution.
From saylordotorg.github.io
Reactions in Aqueous Solution How To Create An Aqueous Solution Decide on the solution's concentration. To understand how and why solutions form. The concentration describes how much solute is in. Describe how an aqueous solution is formed from both ionic compounds. Define what makes an “aqueous solution.” explain the behavior of ionic compounds in water. Sodium hydroxide is an ionic compound that is a strong electrolyte (and a strong base). How To Create An Aqueous Solution.
From www.pinterest.com
saturated unsaturated and supersaturated solutions Google Search How To Create An Aqueous Solution Define a solution and describe the parts of a solution. The solvent in aqueous solutions is water, which. You will need a balance to weigh out the solute and a graduated cylinder to measure the solvent (if it’s water). There are seven steps to making a solution. The solutes are dissolved molecules and ions that are surrounded by water molecules.. How To Create An Aqueous Solution.