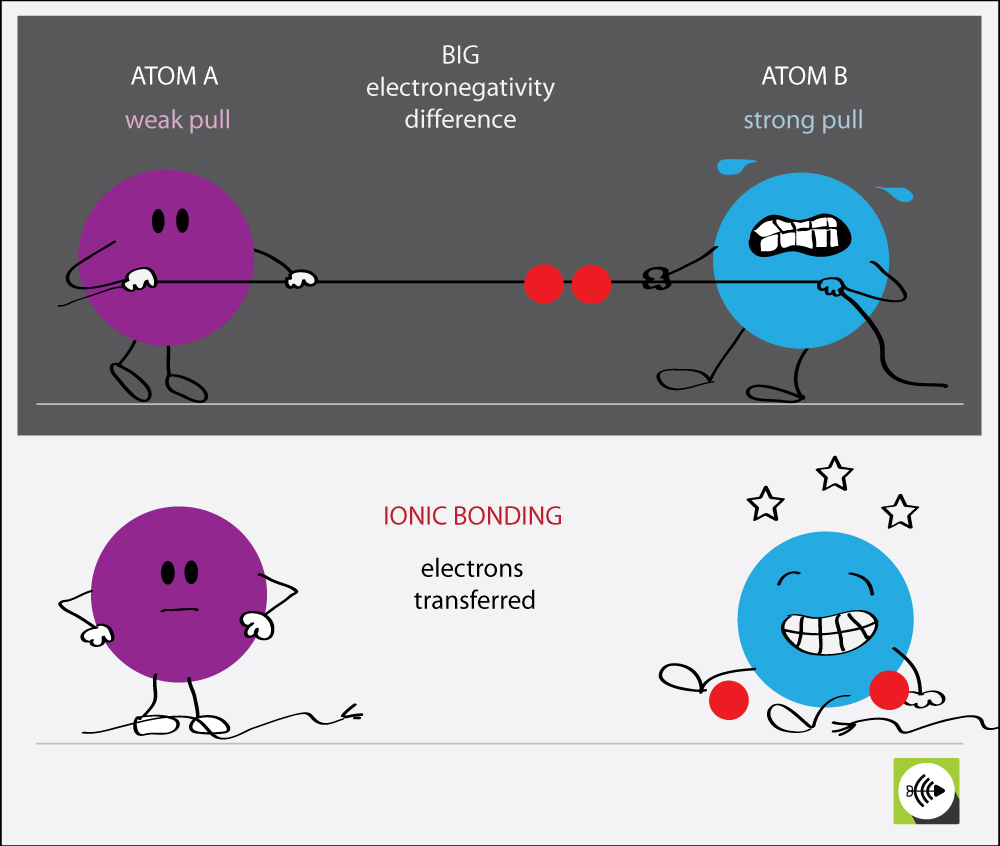
Why Does Krypton Have A High Electronegativity . why do krypton and xenon have high electronegativity? This means they hold on to their electrons tightly, so. In general, an atom’s electronegativity is affected by both its atomic number and the distance. Fluorine is the most electronegative element with an electronegativity value of. the electronegativity of krypton is: contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. what is the element with the highest electronegativity value? elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. In general, an atom’s electronegativity is affected by both its. Noble gases are supposed to be happy with the amount. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. Sources, facts, uses, scarcity (sri), podcasts,. the electronegativity of krypton is:
from surfguppy.com
Noble gases are supposed to be happy with the amount. the electronegativity of krypton is: what is the element with the highest electronegativity value? This means they hold on to their electrons tightly, so. why do krypton and xenon have high electronegativity? In general, an atom’s electronegativity is affected by both its atomic number and the distance. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. Fluorine is the most electronegative element with an electronegativity value of. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. In general, an atom’s electronegativity is affected by both its.
Electronegativity Bond Scale Surfguppy Chemistry made easy for
Why Does Krypton Have A High Electronegativity elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. the electronegativity of krypton is: the electronegativity of krypton is: Sources, facts, uses, scarcity (sri), podcasts,. In general, an atom’s electronegativity is affected by both its atomic number and the distance. what is the element with the highest electronegativity value? In general, an atom’s electronegativity is affected by both its. This means they hold on to their electrons tightly, so. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. Noble gases are supposed to be happy with the amount. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. why do krypton and xenon have high electronegativity? Fluorine is the most electronegative element with an electronegativity value of.
From sciencetrends.com
Electronegativity Chart Science Trends Why Does Krypton Have A High Electronegativity In general, an atom’s electronegativity is affected by both its atomic number and the distance. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. In general, an atom’s electronegativity is affected by both its. Sources, facts, uses, scarcity (sri), podcasts,. elements with a high electronegativity (χ ≥ 2.2 in. Why Does Krypton Have A High Electronegativity.
From bergmannchem.weebly.com
Documents Bergmann's chemistry Why Does Krypton Have A High Electronegativity Sources, facts, uses, scarcity (sri), podcasts,. the electronegativity of krypton is: contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. In general, an atom’s electronegativity is affected by both its. why do krypton and xenon have high electronegativity? the electronegativity of krypton is: Noble gases are supposed. Why Does Krypton Have A High Electronegativity.
From www.numerade.com
SOLVED Arrarge the elements in each set in order increasing Why Does Krypton Have A High Electronegativity Sources, facts, uses, scarcity (sri), podcasts,. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. This means they hold on to their electrons tightly, so. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. In general, an atom’s electronegativity is affected by both its atomic. Why Does Krypton Have A High Electronegativity.
From www.pinterest.com
Periodic Table Electronegativity Trend Ionization energy Why Does Krypton Have A High Electronegativity Fluorine is the most electronegative element with an electronegativity value of. Sources, facts, uses, scarcity (sri), podcasts,. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. In general, an atom’s electronegativity is affected by both its atomic number and the distance. This means they hold on to their electrons tightly,. Why Does Krypton Have A High Electronegativity.
From slideplayer.com
Bond polarity vs. Molecule polarity ppt download Why Does Krypton Have A High Electronegativity elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. In general, an atom’s electronegativity is affected by both its atomic number and the distance. contrary to original thinking, however, krypton has been made to. Why Does Krypton Have A High Electronegativity.
From sciencenotes.org
List of Electronegativity Values of the Elements Why Does Krypton Have A High Electronegativity This means they hold on to their electrons tightly, so. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. the electronegativity of krypton is: Fluorine is the most electronegative element with an electronegativity value of. In general, an atom’s electronegativity is affected by both its. why do krypton. Why Does Krypton Have A High Electronegativity.
From neurotext.library.stonybrook.edu
Cellular Neurophysiology Why Does Krypton Have A High Electronegativity In general, an atom’s electronegativity is affected by both its. Sources, facts, uses, scarcity (sri), podcasts,. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. the electronegativity of krypton is: In general, an atom’s electronegativity is affected by both its atomic number and the distance. the lanthanide contraction. Why Does Krypton Have A High Electronegativity.
From www.myxxgirl.com
Periodic Table Trends Ideas Periodic Table Electrons Electron My XXX Why Does Krypton Have A High Electronegativity what is the element with the highest electronegativity value? Noble gases are supposed to be happy with the amount. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. Sources, facts, uses, scarcity (sri), podcasts,. the electronegativity of krypton is: This means they hold on to their electrons. Why Does Krypton Have A High Electronegativity.
From mungfali.com
Electronegativity Across Periodic Table Why Does Krypton Have A High Electronegativity the electronegativity of krypton is: the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. This means they hold on to their electrons tightly, so. In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: In general, an atom’s electronegativity is affected by both its atomic number and the. Why Does Krypton Have A High Electronegativity.
From www.youtube.com
Lewis Dot Structure for Krypton Atom (Kr) YouTube Why Does Krypton Have A High Electronegativity In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. This means they hold on. Why Does Krypton Have A High Electronegativity.
From www.youtube.com
Element Krypton What is Krypton Element Uses of Krypton YouTube Why Does Krypton Have A High Electronegativity the electronegativity of krypton is: contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. This means they hold on to their electrons tightly, so. Fluorine is the most electronegative element with an electronegativity value of. why do krypton and xenon have high electronegativity? In general, an atom’s electronegativity. Why Does Krypton Have A High Electronegativity.
From mungfali.com
Atom Electronegativity Chart Why Does Krypton Have A High Electronegativity Sources, facts, uses, scarcity (sri), podcasts,. This means they hold on to their electrons tightly, so. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. what is the element with the highest electronegativity value? In. Why Does Krypton Have A High Electronegativity.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Why Does Krypton Have A High Electronegativity In general, an atom’s electronegativity is affected by both its. In general, an atom’s electronegativity is affected by both its atomic number and the distance. the electronegativity of krypton is: Sources, facts, uses, scarcity (sri), podcasts,. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. contrary to original thinking, however, krypton has been. Why Does Krypton Have A High Electronegativity.
From blog.naver.com
유기화학 2장. 극성 공유 결합 산과 염기(1) 네이버 블로그 Why Does Krypton Have A High Electronegativity Noble gases are supposed to be happy with the amount. Sources, facts, uses, scarcity (sri), podcasts,. This means they hold on to their electrons tightly, so. what is the element with the highest electronegativity value? elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. the electronegativity of. Why Does Krypton Have A High Electronegativity.
From valenceelectrons.com
Complete Electron Configuration for Krypton (Kr) Why Does Krypton Have A High Electronegativity why do krypton and xenon have high electronegativity? Noble gases are supposed to be happy with the amount. the electronegativity of krypton is: Sources, facts, uses, scarcity (sri), podcasts,. In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have. Why Does Krypton Have A High Electronegativity.
From www.numerade.com
SOLVED Use the Refcrenccs ACcCs imnorni Hulc if needed for this Why Does Krypton Have A High Electronegativity the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. the electronegativity of krypton is: Sources, facts, uses, scarcity (sri), podcasts,. what is the element with the highest electronegativity value? Fluorine is the most electronegative element with an electronegativity value of. This means they hold on to their electrons tightly, so. contrary to. Why Does Krypton Have A High Electronegativity.
From mungfali.com
Electronegativity Chart And Ionic Character Why Does Krypton Have A High Electronegativity Noble gases are supposed to be happy with the amount. what is the element with the highest electronegativity value? the electronegativity of krypton is: why do krypton and xenon have high electronegativity? This means they hold on to their electrons tightly, so. In general, an atom’s electronegativity is affected by both its. contrary to original thinking,. Why Does Krypton Have A High Electronegativity.
From material-properties.org
Krypton Periodic Table and Atomic Properties Why Does Krypton Have A High Electronegativity what is the element with the highest electronegativity value? In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: This means they hold on to their electrons tightly, so. Fluorine is the most electronegative element with an electronegativity value of. the lanthanide contraction causes these atoms to have a higher than expected. Why Does Krypton Have A High Electronegativity.
From www.vrogue.co
Electronegativity And Its Trend In The Periodic Table vrogue.co Why Does Krypton Have A High Electronegativity This means they hold on to their electrons tightly, so. why do krypton and xenon have high electronegativity? Sources, facts, uses, scarcity (sri), podcasts,. In general, an atom’s electronegativity is affected by both its atomic number and the distance. In general, an atom’s electronegativity is affected by both its. Fluorine is the most electronegative element with an electronegativity value. Why Does Krypton Have A High Electronegativity.
From socratic.org
How does electronegativity change across a period? Socratic Why Does Krypton Have A High Electronegativity In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: Noble gases are supposed to be happy with the amount. Fluorine is the most electronegative element with an electronegativity value of. This means they hold on to their electrons tightly, so. what is the element with the highest electronegativity value? contrary to. Why Does Krypton Have A High Electronegativity.
From www.gauthmath.com
Solved When will a diatomic molecule (i.e., consisting of two atoms Why Does Krypton Have A High Electronegativity Sources, facts, uses, scarcity (sri), podcasts,. Fluorine is the most electronegative element with an electronegativity value of. In general, an atom’s electronegativity is affected by both its. In general, an atom’s electronegativity is affected by both its atomic number and the distance. Noble gases are supposed to be happy with the amount. why do krypton and xenon have high. Why Does Krypton Have A High Electronegativity.
From www.learnatnoon.com
Which Halogen Has The Lowest Electronegativity? Noon Academy Why Does Krypton Have A High Electronegativity why do krypton and xenon have high electronegativity? the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. Noble gases are supposed to be happy with the amount. Sources, facts, uses, scarcity (sri), podcasts,. the electronegativity of krypton is: Fluorine is the most electronegative element with an electronegativity value of. In general, an atom’s. Why Does Krypton Have A High Electronegativity.
From www.animalia-life.club
Atomic Structure Of Krypton Why Does Krypton Have A High Electronegativity In general, an atom’s electronegativity is affected by both its. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. This means they hold on to their electrons tightly, so. why do krypton and xenon have high electronegativity? the electronegativity of krypton is: Sources, facts, uses, scarcity (sri), podcasts,. the electronegativity of krypton. Why Does Krypton Have A High Electronegativity.
From therealgroupichem.weebly.com
Electronegativity Group i Chemistry(iiiescuro) Why Does Krypton Have A High Electronegativity what is the element with the highest electronegativity value? elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. Sources, facts, uses, scarcity (sri), podcasts,. the lanthanide contraction causes. Why Does Krypton Have A High Electronegativity.
From dxofcoqqf.blob.core.windows.net
How Many Valence Electrons Are There In Chlorine at Candace Kelly blog Why Does Krypton Have A High Electronegativity what is the element with the highest electronegativity value? In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. This means they hold on to their electrons tightly, so. Sources, facts, uses, scarcity (sri),. Why Does Krypton Have A High Electronegativity.
From www.aiophotoz.com
Periodic Table Of Elements Electronegativity Chart Periodic Table Why Does Krypton Have A High Electronegativity Noble gases are supposed to be happy with the amount. In general, an atom’s electronegativity is affected by both its. This means they hold on to their electrons tightly, so. the lanthanide contraction causes these atoms to have a higher than expected $z_{\text{eff}}$. the electronegativity of krypton is: In general, an atom’s electronegativity is affected by both its. Why Does Krypton Have A High Electronegativity.
From www.youtube.com
Man of Steel HD Krypton The Phantom Zone' Featurette Why Does Krypton Have A High Electronegativity Noble gases are supposed to be happy with the amount. the electronegativity of krypton is: In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. This means they hold on to their electrons. Why Does Krypton Have A High Electronegativity.
From www.reddit.com
Why do those noble gasses have electronegativity? (Krypton, Xenon, and Why Does Krypton Have A High Electronegativity This means they hold on to their electrons tightly, so. what is the element with the highest electronegativity value? Sources, facts, uses, scarcity (sri), podcasts,. Fluorine is the most electronegative element with an electronegativity value of. In general, an atom’s electronegativity is affected by both its. the electronegativity of krypton is: why do krypton and xenon have. Why Does Krypton Have A High Electronegativity.
From ichigo-kurenai.blogspot.com
Element Krypton Electron Configuration What Is The Ground State Why Does Krypton Have A High Electronegativity why do krypton and xenon have high electronegativity? This means they hold on to their electrons tightly, so. contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. the electronegativity of krypton is: In general, an atom’s electronegativity is affected by both its atomic number and the distance. Fluorine. Why Does Krypton Have A High Electronegativity.
From www.britannica.com
Krypton Properties, Uses, & Facts Britannica Why Does Krypton Have A High Electronegativity Sources, facts, uses, scarcity (sri), podcasts,. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. In general, an atom’s electronegativity is affected by both its. why do krypton and xenon have high electronegativity? This means they hold on to their electrons tightly, so. Noble gases are supposed to. Why Does Krypton Have A High Electronegativity.
From www.sliderbase.com
Periodic Table of Electronegativities Why Does Krypton Have A High Electronegativity This means they hold on to their electrons tightly, so. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. why do krypton and xenon have high electronegativity? In general, an atom’s electronegativity is affected by both its atomic number and the distance. the lanthanide contraction causes these. Why Does Krypton Have A High Electronegativity.
From www.nuclear-power.com
Krypton Electron Affinity Electronegativity Ionization Energy of Why Does Krypton Have A High Electronegativity the electronegativity of krypton is: Noble gases are supposed to be happy with the amount. Fluorine is the most electronegative element with an electronegativity value of. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. contrary to original thinking, however, krypton has been made to react with. Why Does Krypton Have A High Electronegativity.
From valenceelectrons.com
How Many Valence Electrons Does Bromine (Br) Have? Why Does Krypton Have A High Electronegativity the electronegativity of krypton is: In general, an atom’s electronegativity is affected by both its atomic number and the distance. Fluorine is the most electronegative element with an electronegativity value of. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. contrary to original thinking, however, krypton has. Why Does Krypton Have A High Electronegativity.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Why Does Krypton Have A High Electronegativity Fluorine is the most electronegative element with an electronegativity value of. why do krypton and xenon have high electronegativity? contrary to original thinking, however, krypton has been made to react with the highly electronegative elements and is. the electronegativity of krypton is: In general, an atom’s electronegativity is affected by both its. Noble gases are supposed to. Why Does Krypton Have A High Electronegativity.