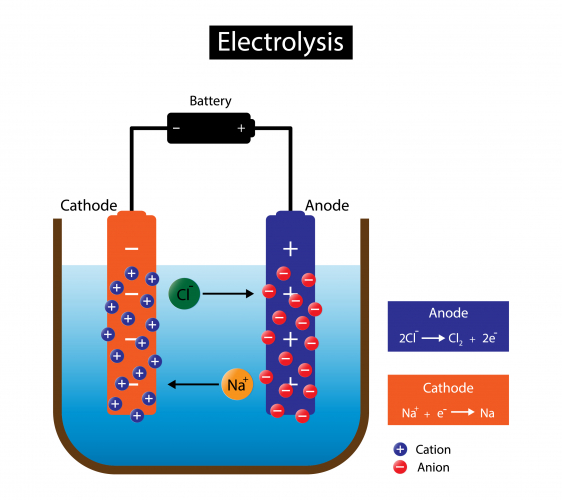
Reactions At Electrodes During Electrolysis . During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. The electrolysis of aqueous sodium chloride. When aqueous solutions of ionic compounds are electrolyzed, the anode and.
from www.edplace.com
Ionic compounds conduct electricity when molten or in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Reactive metals are extracted from their ores using electrolysis. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. When aqueous solutions of ionic compounds are electrolyzed, the anode and. The electrolysis of aqueous sodium chloride. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution.
Understand How Electrolysis Works Worksheet EdPlace
Reactions At Electrodes During Electrolysis Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. Ionic compounds conduct electricity when molten or in solution. When aqueous solutions of ionic compounds are electrolyzed, the anode and. Reactive metals are extracted from their ores using electrolysis. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. The electrolysis of aqueous sodium chloride.
From www.chemistry-teaching-resources.com
chemistry picture Reactions At Electrodes During Electrolysis As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the. Reactions At Electrodes During Electrolysis.
From www.researchgate.net
Pathway of a general electrode reaction (see [27]). Download Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Reactive metals are extracted from their ores using electrolysis. When aqueous solutions of ionic compounds are electrolyzed, the anode and. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical. Reactions At Electrodes During Electrolysis.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner Reactions At Electrodes During Electrolysis Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. Ionic compounds conduct electricity. Reactions At Electrodes During Electrolysis.
From www.researchgate.net
Schematic of the electrodeelectrolyte interface in electrochemical Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Reactive metals are extracted from their ores using electrolysis. The electrolysis of aqueous sodium chloride. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During the electrolysis, hydrogen and chloride ions are removed. Reactions At Electrodes During Electrolysis.
From circuitlistjames.z21.web.core.windows.net
Electrolytic Cell Schematic Diagram Reactions At Electrodes During Electrolysis During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. As the ions come into contact with the electrode, electrons are either lost or gained and they form. Reactions At Electrodes During Electrolysis.
From www.researchgate.net
Schematic diagram of the electrode reactions for the electrodes with Reactions At Electrodes During Electrolysis When aqueous solutions of ionic compounds are electrolyzed, the anode and. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. The electrolysis of aqueous sodium chloride. Ionic compounds. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS A guide for GCSE students PowerPoint Presentation Reactions At Electrodes During Electrolysis In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Reactive metals are extracted from their ores using electrolysis. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. The electrolysis of aqueous sodium chloride. When aqueous solutions of ionic compounds are electrolyzed,. Reactions At Electrodes During Electrolysis.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Reactions At Electrodes During Electrolysis Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Reactive metals are. Reactions At Electrodes During Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Reactions At Electrodes During Electrolysis In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. When aqueous solutions of ionic compounds are electrolyzed, the anode and. Reactive metals are extracted from their ores using electrolysis. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral. Reactions At Electrodes During Electrolysis.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Reactions At Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. The electrolysis of aqueous sodium chloride. Predict the product formed. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT Secondary 4 Chemistry Extraction of Aluminium via electrolysis JT Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. When aqueous solutions of ionic compounds are electrolyzed, the anode and. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. In this chapter, we have described various galvanic. Reactions At Electrodes During Electrolysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Reactions At Electrodes During Electrolysis Ionic compounds conduct electricity when molten or in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. The electrolysis of aqueous sodium chloride. When aqueous solutions of ionic compounds are electrolyzed, the anode and. During electrolysis, positively charged ions move to the negative electrode (cathode) , and. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Reactions At Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. When aqueous solutions of ionic compounds are electrolyzed, the anode and. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. The electrolysis of aqueous sodium chloride. In this chapter, we have described various galvanic cells in which a spontaneous chemical. Reactions At Electrodes During Electrolysis.
From www.researchgate.net
Distribution of reactant concentration near electrode during Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Reactive metals are extracted from their ores using electrolysis. When aqueous solutions of ionic compounds are electrolyzed, the anode and. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. When aqueous solutions of ionic compounds are electrolyzed, the anode and. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Reactive metals are extracted from their ores using. Reactions At Electrodes During Electrolysis.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Reactions At Electrodes During Electrolysis In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Reactions At Electrodes During Electrolysis As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. When aqueous solutions of ionic compounds are electrolyzed, the anode and. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium. Reactions At Electrodes During Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Reactions At Electrodes During Electrolysis As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. The electrolysis of aqueous sodium chloride. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Reactions At Electrodes During Electrolysis The electrolysis of aqueous sodium chloride. When aqueous solutions of ionic compounds are electrolyzed, the anode and. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Reactive metals are extracted from their ores. Reactions At Electrodes During Electrolysis.
From courses.lumenlearning.com
Electrolysis Chemistry Reactions At Electrodes During Electrolysis The electrolysis of aqueous sodium chloride. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Ionic compounds conduct electricity when molten or in solution. As the ions come into contact with the. Reactions At Electrodes During Electrolysis.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry Reactions At Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. Ionic compounds conduct electricity when molten or in solution. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. When aqueous. Reactions At Electrodes During Electrolysis.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube Reactions At Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Ionic compounds conduct electricity when molten or in solution. When aqueous solutions of ionic compounds are electrolyzed, the anode and. Predict the product formed at the positive electrode during the. Reactions At Electrodes During Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson Reactions At Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. Ionic compounds conduct electricity when molten or in solution. As the ions come into. Reactions At Electrodes During Electrolysis.
From www.researchgate.net
1 Pathway of a general electrode reaction. Download Scientific Diagram Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. When aqueous solutions of ionic compounds are electrolyzed, the anode and. The electrolysis of aqueous sodium chloride. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Reactive metals. Reactions At Electrodes During Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Reactions At Electrodes During Electrolysis Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. When aqueous solutions of ionic compounds are electrolyzed, the anode and. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction. Reactions At Electrodes During Electrolysis.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Reactions At Electrodes During Electrolysis As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. When aqueous solutions of ionic compounds are electrolyzed, the anode and. Ionic compounds conduct electricity when molten or in. Reactions At Electrodes During Electrolysis.
From revisechemistry.uk
Electrolysis AQA C4 revisechemistry.uk Reactions At Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. The electrolysis of aqueous sodium chloride. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Reactions At Electrodes During Electrolysis When aqueous solutions of ionic compounds are electrolyzed, the anode and. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Ionic compounds conduct electricity when molten or in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical. Reactions At Electrodes During Electrolysis.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. The electrolysis of aqueous sodium chloride. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and. Reactions At Electrodes During Electrolysis.
From slidetodoc.com
Define electrolysis The breakdown of an ionic compound Reactions At Electrodes During Electrolysis During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. When aqueous solutions of ionic compounds are electrolyzed, the anode and. Ionic compounds conduct electricity when molten or in solution. During the electrolysis, hydrogen. Reactions At Electrodes During Electrolysis.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry Reactions At Electrodes During Electrolysis During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Ionic compounds conduct electricity when molten or in solution. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. When aqueous solutions of ionic compounds are electrolyzed, the. Reactions At Electrodes During Electrolysis.
From www.researchgate.net
Pathway of a general electrode reaction. Download Scientific Diagram Reactions At Electrodes During Electrolysis Reactive metals are extracted from their ores using electrolysis. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Predict the product formed at the positive electrode during the electrolysis of concentrated sodium sulfate solution. The electrolysis of aqueous sodium chloride. In this chapter, we have described various galvanic. Reactions At Electrodes During Electrolysis.
From en.ppt-online.org
Electrolysis online presentation Reactions At Electrodes During Electrolysis When aqueous solutions of ionic compounds are electrolyzed, the anode and. The electrolysis of aqueous sodium chloride. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. As the. Reactions At Electrodes During Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Reactions At Electrodes During Electrolysis Ionic compounds conduct electricity when molten or in solution. As the ions come into contact with the electrode, electrons are either lost or gained and they form neutral substances. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. Predict the product formed at the positive electrode during the. Reactions At Electrodes During Electrolysis.
From www.slideserve.com
PPT Lecture 2. Basic Electrochemistry PowerPoint Presentation, free Reactions At Electrodes During Electrolysis Ionic compounds conduct electricity when molten or in solution. During the electrolysis, hydrogen and chloride ions are removed from solution whereas sodium and hydroxide ions are left behind in solution. Reactive metals are extracted from their ores using electrolysis. During electrolysis, positively charged ions move to the negative electrode (cathode) , and negatively charged ions move to the positive. As. Reactions At Electrodes During Electrolysis.