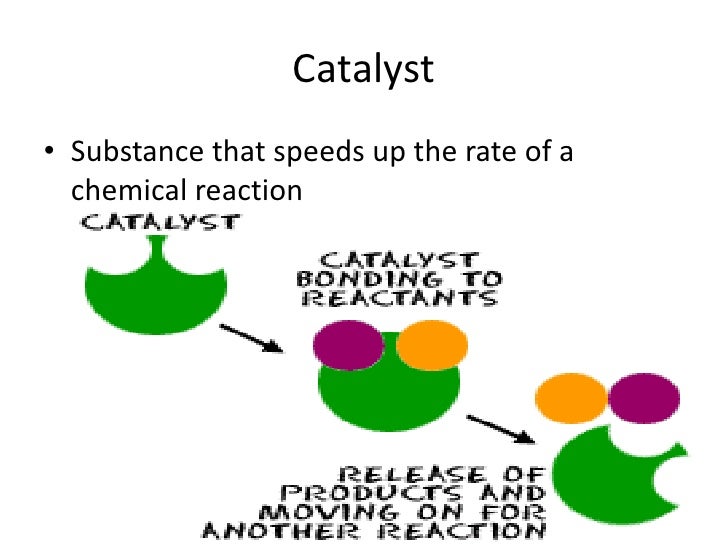
Catalyst Definition Rate . A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. A catalyst does not actually become part of the products of. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn how catalysts work, their types and examples, and. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn about positive and negative catalysts, promoters, catalytic poisons,.
from www.slideshare.net
A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a chemical substance. Learn how catalysts work, their types and examples, and. Learn about the three major classes of. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Learn about positive and negative catalysts, promoters, catalytic poisons,. A catalyst does not actually become part of the products of. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy.
Biology 2.4
Catalyst Definition Rate A catalyst is a chemical substance. A catalyst is a chemical substance. A catalyst does not actually become part of the products of. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn about the three major classes of. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn about positive and negative catalysts, promoters, catalytic poisons,. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn how catalysts work, their types and examples, and. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Catalyst Definition Rate Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn how catalysts work, their types and examples, and. A catalyst is a chemical substance. Learn about positive and negative. Catalyst Definition Rate.
From spmchemistry.blog.onlinetuition.com.my
Factors Affecting the Rate of Reaction SPM Chemistry Catalyst Definition Rate A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn how catalysts work, their types and examples, and. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Catalysis is the process that alters the rate of a chemical reaction under. Catalyst Definition Rate.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Definition Rate Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a chemical substance. Learn about positive and negative catalysts, promoters, catalytic poisons,. Learn how catalysts. Catalyst Definition Rate.
From www.pnnl.gov
Catalysis PNNL Catalyst Definition Rate A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Catalysis is the process of using substances that increase the reaction rate of. Catalyst Definition Rate.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Definition Rate Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Learn about positive and negative catalysts, promoters, catalytic poisons,. Learn about the three major classes of. Enzymes are. Catalyst Definition Rate.
From study.com
Effect of Catalysts on Rates of Reaction Video & Lesson Transcript Catalyst Definition Rate Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn about the three major classes of. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn how catalysts work, their types and examples, and. A catalyst is a substance that affects the rate of. Catalyst Definition Rate.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Definition Rate Learn about the three major classes of. Learn how catalysts work, their types and examples, and. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysis is. Catalyst Definition Rate.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalyst Definition Rate A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process.. Catalyst Definition Rate.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Catalyst Definition Rate Learn how catalysts work, their types and examples, and. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a chemical substance. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that affects. Catalyst Definition Rate.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Definition Rate A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Enzymes are naturally occurring. Catalyst Definition Rate.
From www.waca.msf.org
Catalyst, Catalyst Catalyst Definition Rate Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. Learn about the three major classes of. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. A catalyst is a substance that can help the. Catalyst Definition Rate.
From sheetalschemblog.blogspot.com.tr
Sheetal's Chemistry Blog Catalyst Definition Rate A catalyst is a chemical substance. Learn about positive and negative catalysts, promoters, catalytic poisons,. A catalyst does not actually become part of the products of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that can help the reactants in a. Catalyst Definition Rate.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Definition Rate A catalyst does not actually become part of the products of. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that affects the rate of a chemical reaction by lowering. Catalyst Definition Rate.
From www.rankred.com
What Is A Catalyst? Definition Types Examples RankRed Catalyst Definition Rate A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn about positive and negative catalysts, promoters, catalytic poisons,. Catalyst, in chemistry, any substance. Catalyst Definition Rate.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Definition Rate Learn about positive and negative catalysts, promoters, catalytic poisons,. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn about the three major classes of. A catalyst is a substance that. Catalyst Definition Rate.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Definition Rate Learn how catalysts work, their types and examples, and. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Catalysis is the process that alters the rate of a chemical reaction under the influence of. Catalyst Definition Rate.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Catalyst Definition Rate A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process.. Catalyst Definition Rate.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Definition Rate A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Enzymes are naturally occurring catalysts responsible for many. A catalyst does not actually become part of the products of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn. Catalyst Definition Rate.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Definition Rate A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn about the three major classes. Catalyst Definition Rate.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Definition Rate A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a chemical substance. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn how catalysts work, their types and examples, and. Learn about the. Catalyst Definition Rate.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Definition Rate A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a chemical substance. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that changes the rate of a chemical reaction without. Catalyst Definition Rate.
From www.slideshare.net
Biology 2.4 Catalyst Definition Rate A catalyst does not actually become part of the products of. Learn about positive and negative catalysts, promoters, catalytic poisons,. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. A catalyst is a chemical substance. Catalysis is the process of using substances that increase the reaction rate of a. Catalyst Definition Rate.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Catalyst Definition Rate Learn about the three major classes of. A catalyst is a chemical substance. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that changes the rate. Catalyst Definition Rate.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Definition Rate Learn how catalysts work, their types and examples, and. A catalyst does not actually become part of the products of. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that affects the rate of a chemical reaction by lowering its. Catalyst Definition Rate.
From www.slideserve.com
PPT Rates of Reactions Part 2 PowerPoint Presentation, free Catalyst Definition Rate A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalysis is the process that alters the rate of a chemical. Catalyst Definition Rate.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Definition Rate Learn about positive and negative catalysts, promoters, catalytic poisons,. A catalyst does not actually become part of the products of. Learn how catalysts work, their types and examples, and. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process that alters the. Catalyst Definition Rate.
From www.slideshare.net
chemistryenthalpy power point Catalyst Definition Rate Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalyst Definition Rate.
From ppt-online.org
Rates of reaction презентация онлайн Catalyst Definition Rate A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the. Catalyst Definition Rate.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Definition Rate Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that changes the rate of a chemical reaction. Catalyst Definition Rate.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID Catalyst Definition Rate A catalyst does not actually become part of the products of. Learn about the three major classes of. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Learn about positive and negative catalysts, promoters, catalytic poisons,. A catalyst is a chemical substance. Catalysis is the process that alters the rate. Catalyst Definition Rate.
From gamesmartz.com
Catalyst Definition & Image GameSmartz Catalyst Definition Rate Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst does not actually become part of the products of. A catalyst is a chemical substance. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation. Catalyst Definition Rate.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Definition Rate Learn about positive and negative catalysts, promoters, catalytic poisons,. A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Learn how catalysts work, their types and examples, and. Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical. Catalyst Definition Rate.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction Catalyst Definition Rate Learn about positive and negative catalysts, promoters, catalytic poisons,. Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn how catalysts work, their types. Catalyst Definition Rate.
From www.scribd.com
Catalyst Definition PDF Catalysis Reaction Rate Catalyst Definition Rate A catalyst is a substance that changes the rate of a chemical reaction without being used up in the reaction. Learn how catalysts work, their types and examples, and. A catalyst is a chemical substance. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance that can. Catalyst Definition Rate.
From www.slideserve.com
PPT CATALYSIS PowerPoint Presentation, free download ID270211 Catalyst Definition Rate A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process of using substances that increase the reaction rate of a chemical. Catalyst Definition Rate.