Osmometer Calculation . Osmolality is a standard measure of the concentration of particles in a solute. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. It is only based on their number, not on the nature of molecules. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum.
from www.chegg.com
The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. It is only based on their number, not on the nature of molecules. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration.
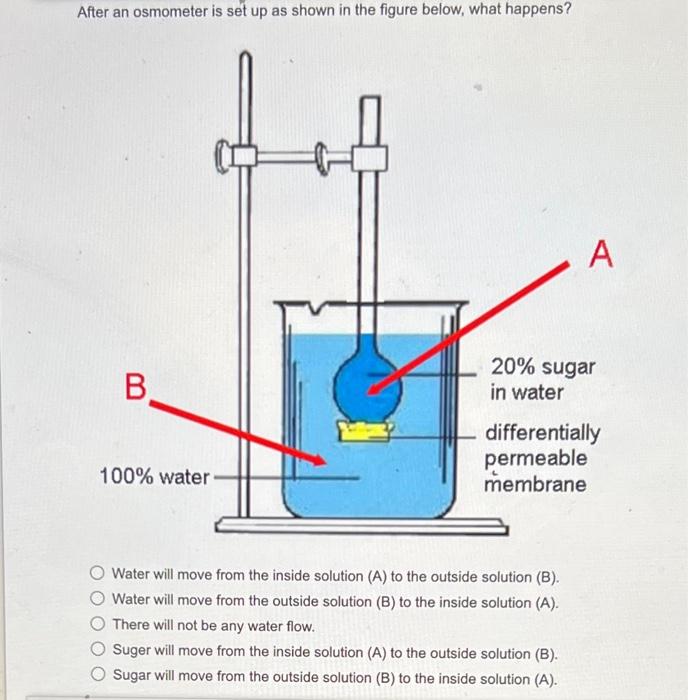
Solved after an osmometer is set up as shown in the figure
Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. It is only based on their number, not on the nature of molecules. Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum.
From americanlaboratorytrading.com
Advanced Instruments Model 3250 SingleSample Osmometer Osmometer Calculation The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. It is only based on their number, not on the nature of molecules. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. Osmolality is a standard measure. Osmometer Calculation.
From www.slideserve.com
PPT Chapter 2. Molecular Weight and Polymer Solutions PowerPoint Osmometer Calculation An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. It is only based on their number, not on the nature of molecules. Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution,. Osmometer Calculation.
From testbook.com
Understanding Osmosis Using Potato Osmometer Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. It is only based on their number, not on the nature of molecules. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. An osmometer is a device for measuring the osmotic strength of. Osmometer Calculation.
From usedlabequipment.com
Advanced Instruments Model 3320 MicroOsmometer Osmometers, Osmometers Osmometer Calculation It is only based on their number, not on the nature of molecules. Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. An osmometer is a device for measuring the osmotic strength of. Osmometer Calculation.
From studylib.net
Membrane Osmometry ( , A , χ) M 2 Osmometer Calculation An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. Osmolality is. Osmometer Calculation.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the Osmometer Calculation It is only based on their number, not on the nature of molecules. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. The serum osmolality/osmolarity calculates expected serum osmolarity, for. Osmometer Calculation.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometer Calculation An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a 2, χ) •. Osmometer Calculation.
From www.biophlox.com
A Complete Lab Guide to Osmometer Osmometer Calculation The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. Osmolality is a standard measure of the concentration of particles in a solute. The salts of sodium (choloride. Osmometer Calculation.
From acmerevival.com
Advanced Instruments 3300 Micro Osmometer FOR SALE Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. The. Osmometer Calculation.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Osmometer Calculation The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a. Osmometer Calculation.
From dir.indiamart.com
Osmometer at Best Price in India Osmometer Calculation An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. It is. Osmometer Calculation.
From www.geminibv.com
Advanced Instruments 3320 Osmometer Gemini BV Osmometer Calculation An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. It is only based on their number, not on the nature of molecules. An osmometer is a device used in clinical laboratories for measuring the. Osmometer Calculation.
From www.concordscientificdevices.com
vaporpressureosmometer Osmometer Calculation An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. It is only based on their number, not on the nature of molecules. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. Osmolality is a standard measure. Osmometer Calculation.
From www.purechemistry.org
Osmometry method to determine molecular weight of polymer Purechemistry Osmometer Calculation An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is. Osmometer Calculation.
From www.dotmed.com
Osmometer For Sale or Wanted Osmometer Calculation It is only based on their number, not on the nature of molecules. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device used in clinical laboratories for measuring the concentration of particles. Osmometer Calculation.
From dir.indiamart.com
Osmometer Analytical Osmometer Latest Price, Manufacturers & Suppliers Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. It is only based on their number, not on the nature of molecules. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of. Osmometer Calculation.
From www.researchgate.net
The osmometer properties of swelling protoplasts. A, a, An Osmometer Calculation The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. It is only based on their number, not on the nature of molecules. An osmometer is a device for measuring the osmotic. Osmometer Calculation.
From www.academia.edu
(PDF) Osmotic Pressure Measurement via Freezing Point Osmometer and Osmometer Calculation An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. Osmolality is a standard measure of the concentration of particles in a solute. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. The serum osmolality/osmolarity calculates expected. Osmometer Calculation.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometer Calculation The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is a standard measure of the concentration of particles in a solute. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. It is only based on their number, not on. Osmometer Calculation.
From www.slideserve.com
PPT Labs 6 & 7 PowerPoint Presentation, free download ID2180146 Osmometer Calculation The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. The serum osmolality/osmolarity calculates expected. Osmometer Calculation.
From shop.unigreenscheme.co.uk
Advanced Instruments Model3D3 Single Sample Osmometer Osmometer Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. It is only based on their number, not on the nature of. Osmometer Calculation.
From www.seoulin.co.kr
[ADVANCED INSTRUMENTS] Osmometer 삼투압 장비 서린바이오사이언스 Osmometer Calculation An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device. Osmometer Calculation.
From nanditamaqiyas.blogspot.com
Protein Molecular Weight Calculation Osmometer Calculation The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. It is only based on their number, not on the nature of molecules. An osmometer is a device used in clinical laboratories for measuring the. Osmometer Calculation.
From www.tokopedia.com
Jual Osmometer Osmospecial 1 Osmometer Jakarta Barat Digitalgrp Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. It is only based on their number, not on the nature of molecules. An osmometer is a device used in clinical laboratories for measuring the concentration of particles. Osmometer Calculation.
From mx.vwr.com
Advanced® MicroOsmometer, Model 3320, Advanced Instruments VWR Osmometer Calculation An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. Osmolality is a standard measure of the concentration of particles in a solute. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. It is only based on. Osmometer Calculation.
From americanlaboratorytrading.com
Refurbished Advanced Instruments Model 3320 MicroOsmometer Osmometer Calculation The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the. Osmometer Calculation.
From www.indiamart.com
Fiske 210 Micro Sample Osmometer at Rs 761000 North West New Delhi Osmometer Calculation It is only based on their number, not on the nature of molecules. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. Membrane osmometry ( , a 2, χ) •. Osmometer Calculation.
From slidetodoc.com
Membrane Osmometry Alfredo Clemente CH 392 N Prof Osmometer Calculation Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. It is only based on their number, not on the nature of molecules. An osmometer is a device. Osmometer Calculation.
From americanlaboratorytrading.com
ALT ITEM 31905 Single Sample Osmometer, Model 3D3 Osmometer Calculation The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of. Osmometer Calculation.
From www.geminibv.com
Advanced instruments inc. 3300 Micro Osmometer Gemini BV Osmometer Calculation The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. It is only based on their number, not on the nature of molecules. Osmolality is a standard measure of the concentration of. Osmometer Calculation.
From www.chegg.com
Solved after an osmometer is set up as shown in the figure Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. The salts of sodium (choloride. Osmometer Calculation.
From www.youtube.com
How to solve osmolarity calculation problems YouTube Osmometer Calculation It is only based on their number, not on the nature of molecules. Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. An osmometer is a device used in clinical laboratories for measuring. Osmometer Calculation.
From hylandscientific.com
Advanced Instruments Model Osmo1 Osmometer with Accessories Hyland Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. The salts of sodium (choloride. Osmometer Calculation.
From www.il-biosystems.com
OsmoPRO MAX Osmometer Efficient Osmolality Determination Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. It is only based on their number, not on the nature of molecules. Membrane osmometry ( , a 2, χ) • osmotic pressure, π,. Osmometer Calculation.
From www.youtube.com
Calculate Molecular Weight from Osmotic Pressure for Nonideal Solution Osmometer Calculation Osmolality is a standard measure of the concentration of particles in a solute. Membrane osmometry ( , a 2, χ) • osmotic pressure, π, is a colligative property which depends only on the number of solute. The serum osmolality/osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum. It is only based on. Osmometer Calculation.