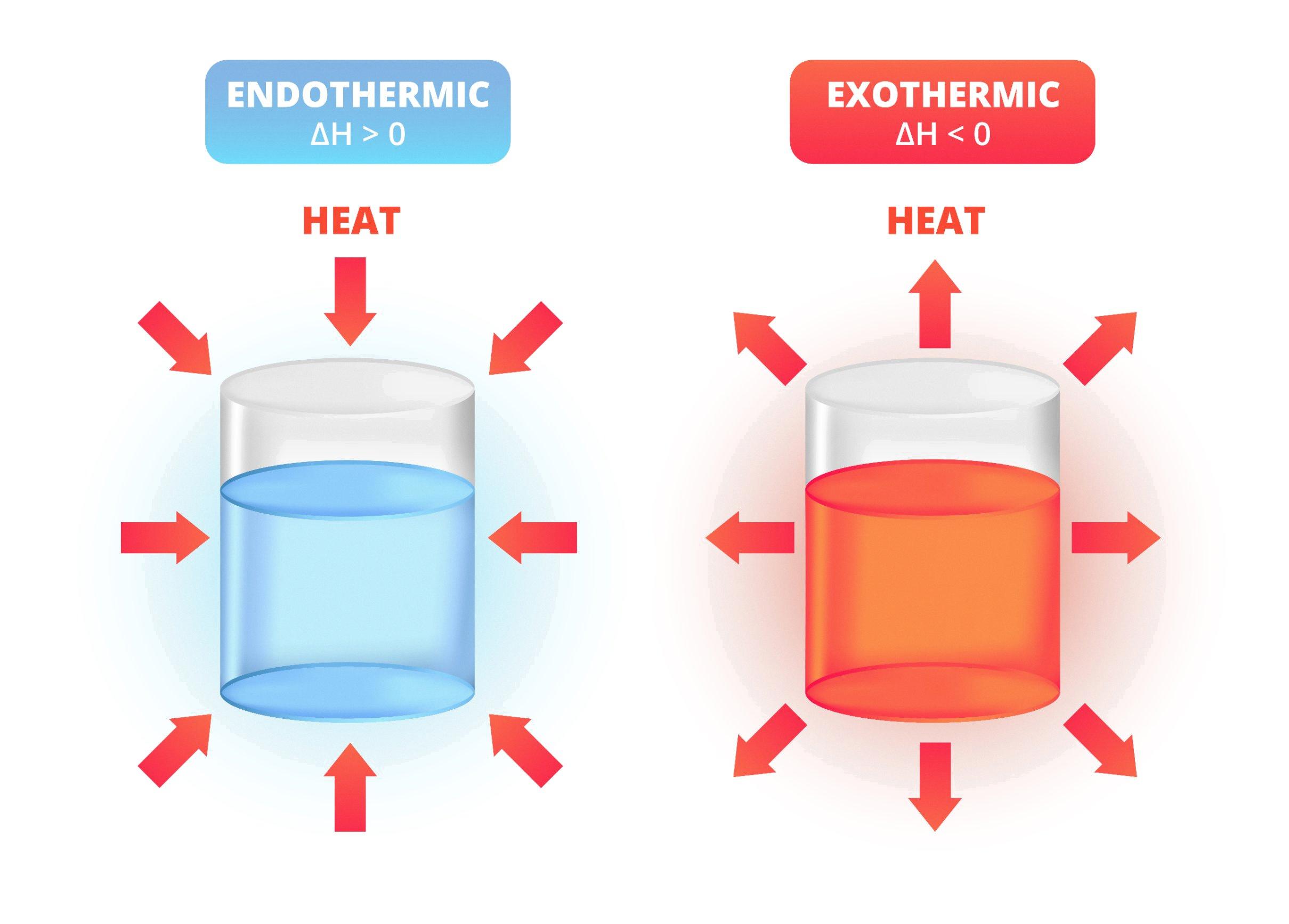
Endothermic Reaction Positive Or Negative . chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. This can be used to classify. An endothermic reaction is a. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to.
from h-o-m-e.org
endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. An endothermic reaction is a. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. This can be used to classify. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to.
Endothermic Reactions The Science Behind Temperature Change
Endothermic Reaction Positive Or Negative This can be used to classify. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. This can be used to classify. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. An endothermic reaction is a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product.
From www.dreamstime.com
Exothermic and Endothermic Reactions in Chemistry Stock Illustration Endothermic Reaction Positive Or Negative the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An endothermic reaction is a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reactions are characterized by positive heat flow (into the reaction). Endothermic Reaction Positive Or Negative.
From byjus.com
which of the following reaction is said to be entropy driven 1 Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. endothermic reactions are characterized by positive heat flow (into the. Endothermic Reaction Positive Or Negative.
From www.vrogue.co
Exothermic And Endothermic Processes Introduction To vrogue.co Endothermic Reaction Positive Or Negative This can be used to classify. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. An endothermic reaction is a. examples of endothermic reactions include photosynthesis, dissolving salt in water, and. Endothermic Reaction Positive Or Negative.
From www.vrogue.co
Differentiate Between Exothermic And Endothermic Reac vrogue.co Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical. Endothermic Reaction Positive Or Negative.
From educationsquish.z13.web.core.windows.net
How Does Temperature Affect Spontaneity Endothermic Reaction Positive Or Negative the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the. Endothermic Reaction Positive Or Negative.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Endothermic Reaction Positive Or Negative the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or. Endothermic Reaction Positive Or Negative.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a. This can be used to classify. the changes in energy that. Endothermic Reaction Positive Or Negative.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Positive Or Negative an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. examples of. Endothermic Reaction Positive Or Negative.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Positive Or Negative examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). This can be used to classify. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. the changes in energy. Endothermic Reaction Positive Or Negative.
From general.chemistrysteps.com
The Effect of 𝚫H, 𝚫S, and T on 𝚫G Spontaneity Chemistry Steps Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. An endothermic reaction is a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reactions are characterized. Endothermic Reaction Positive Or Negative.
From thechemistrynotes.com
Differences Between Exothermic and Endothermic Reactions Endothermic Reaction Positive Or Negative endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. examples of endothermic reactions include photosynthesis, dissolving salt in. Endothermic Reaction Positive Or Negative.
From slideplayer.com
Collision Theory of Reactions ppt download Endothermic Reaction Positive Or Negative the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. if δh is positive. Endothermic Reaction Positive Or Negative.
From slideplayer.com
Learning Objective Describe energy transfers during a reaction ppt Endothermic Reaction Positive Or Negative chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. This can be used to classify. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of. Endothermic Reaction Positive Or Negative.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. examples of endothermic reactions include photosynthesis, dissolving salt in water,. Endothermic Reaction Positive Or Negative.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Positive Or Negative examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of. Endothermic Reaction Positive Or Negative.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.4 Energy Level Diagrams翰林国际教育 Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. This can be used to classify. An endothermic reaction is a. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. an endothermic reaction occurs. Endothermic Reaction Positive Or Negative.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Positive Or Negative the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. endothermic and exothermic reactions can be thought. Endothermic Reaction Positive Or Negative.
From brainly.com
How to tell if a reaction is exothermic or endothermic from an equation Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in energy that occur during a chemical reaction can be seen. Endothermic Reaction Positive Or Negative.
From www.twinkl.ae
What is a Reaction Profile? Answered Twinkl Twinkl Endothermic Reaction Positive Or Negative endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in. Endothermic Reaction Positive Or Negative.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Positive Or Negative endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). An. Endothermic Reaction Positive Or Negative.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Positive Or Negative chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). An endothermic reaction is a. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. examples. Endothermic Reaction Positive Or Negative.
From www.ck12.org
Potential Energy Diagrams ( Read ) Chemistry CK12 Foundation Endothermic Reaction Positive Or Negative an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. This can be used to classify. if δh is positive (+) then the chemical reaction is endothermic, because. Endothermic Reaction Positive Or Negative.
From www.studypool.com
SOLUTION 36 exothermic and endothermic reactions pptx Studypool Endothermic Reaction Positive Or Negative This can be used to classify. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. chemical reactions are categorized. Endothermic Reaction Positive Or Negative.
From circuitwiringtray.z13.web.core.windows.net
Endothermic Enthalpy Diagram Endothermic Reaction Positive Or Negative An endothermic reaction is a. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. if δh is positive (+) then the chemical reaction is endothermic, because less energy is. Endothermic Reaction Positive Or Negative.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in energy that occur during a chemical reaction can be seen. Endothermic Reaction Positive Or Negative.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction Positive Or Negative the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An endothermic reaction is a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. This can be used to classify. if δh is positive (+). Endothermic Reaction Positive Or Negative.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Positive Or Negative chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed. Endothermic Reaction Positive Or Negative.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Positive Or Negative This can be used to classify. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. An endothermic reaction is a. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. the changes in energy that occur during a chemical reaction can be seen. Endothermic Reaction Positive Or Negative.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Reaction Positive Or Negative endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. This can be used to classify. chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. . Endothermic Reaction Positive Or Negative.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Positive Or Negative if δh is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in energy that occur during a chemical reaction can be seen. Endothermic Reaction Positive Or Negative.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Positive Or Negative endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. if δh is positive. Endothermic Reaction Positive Or Negative.
From www.buzzle.com
Endothermic Reaction Examples Endothermic Reaction Positive Or Negative endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This can. Endothermic Reaction Positive Or Negative.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Positive Or Negative endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. This can be used to classify. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. if δh is positive (+) then the chemical reaction is endothermic, because. Endothermic Reaction Positive Or Negative.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic Vs Exothermic Chemical Reactions Endothermic Reaction Positive Or Negative endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. if δh is positive. Endothermic Reaction Positive Or Negative.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Positive Or Negative chemical reactions are categorized as either endothermic, with a change in positive energy, or exothermic,. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. An endothermic reaction is a. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical. Endothermic Reaction Positive Or Negative.