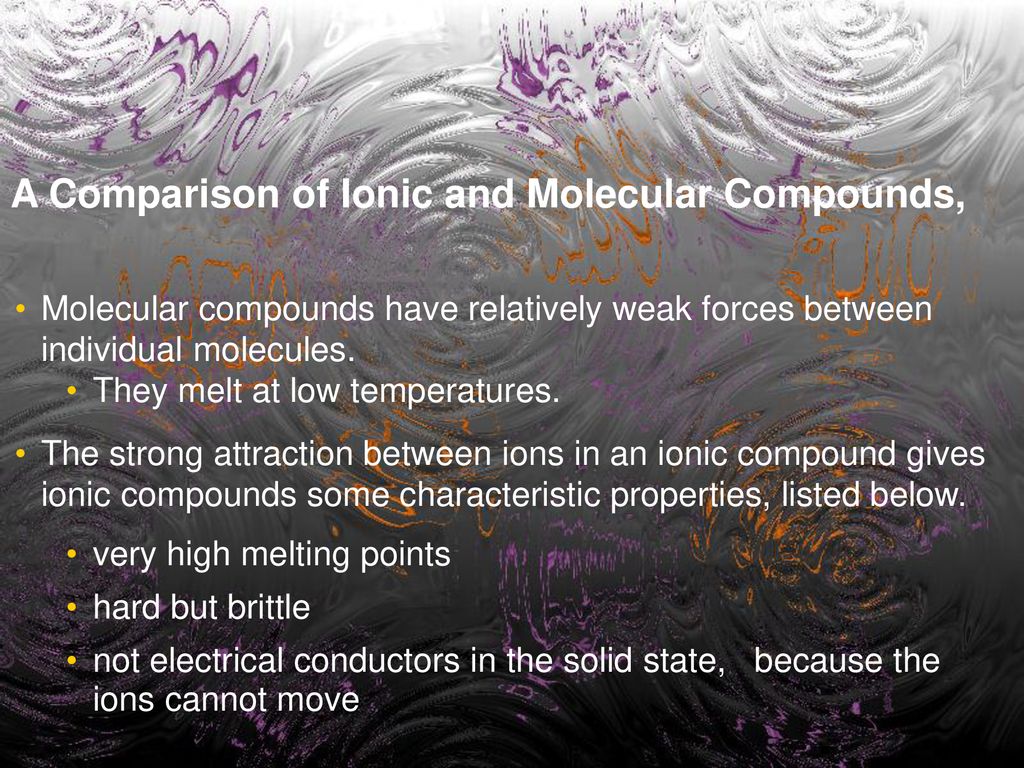
Ionic Compounds Brittle Because . It takes a large amount of mechanical force, such as striking a crystal. However, when that happens, it brings ions of the same charge next to each other (see below). When an external force is applied, it can shift the layers of ions, causing. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are strong and hard because of the strong. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Explain why most ionic compounds are strong and hard, yet brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are generally hard, but brittle. See the study guide on the.
from slideplayer.com
Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle because they have a crystal lattice structure. See the study guide on the. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds are generally hard, but brittle.
Chemical Bonds. ppt download
Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. When an external force is applied, it can shift the layers of ions, causing. In summary, brittleness is not a property uniquely associated with ionic compounds. However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds are strong and hard because of the strong. Ionic compounds are generally hard, but brittle. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. See the study guide on the. Ionic compounds are brittle because they have a crystal lattice structure. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical force, such as striking a crystal.
From slideplayer.com
Notes Ionic Bonds 1. Key Concept Ionic bonds form when electrons are Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard because of the strong. When an external force is applied, it can shift the layers of ions, causing. It takes a large amount. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Ionic Compounds Brittle Because Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. When an external force is applied, it can shift the layers of ions, causing. It. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic Bonding. ppt download Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. See the study guide on the. Ionic compounds are brittle because they have a crystal lattice structure. However, when that happens, it brings ions of the same charge next to each other (see below). It takes a large amount of mechanical force, such as striking a. Ionic Compounds Brittle Because.
From slideplayer.com
WRITING AND NAMING IONIC COMPOUNDS ppt download Ionic Compounds Brittle Because See the study guide on the. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. However, when that happens, it brings ions of the same charge next to each other (see below). It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID5979988 Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. Ionic compounds are brittle because they have a crystal lattice structure. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are strong and hard because of the strong. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. It takes a large. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic Bonding/Naming. ppt download Ionic Compounds Brittle Because Explain why most ionic compounds are strong and hard, yet brittle. When an external force is applied, it can shift the layers of ions, causing. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. In summary, brittleness is not a property uniquely associated with ionic compounds. See the study guide on the.. Ionic Compounds Brittle Because.
From www.studocu.com
Ionic bonds ionic bonds brittle substances with high melting points Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. See the study guide on the. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are strong and hard because of the strong. Explain why. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. See the study guide on the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. In summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds are brittle. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic Bonds. ppt download Ionic Compounds Brittle Because When an external force is applied, it can shift the layers of ions, causing. However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are strong and hard because of the. Ionic Compounds Brittle Because.
From slideplayer.com
Chapter 6 Section 1 Compounds and Molecules ppt download Ionic Compounds Brittle Because Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle because they have a crystal lattice structure. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation Ionic Compounds Brittle Because However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds have high melting and boiling points,. Ionic Compounds Brittle Because.
From www.youtube.com
Why ionic solids are brittle? YouTube Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. When an external force is applied, it can shift the layers of ions, causing. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Ionic Compounds Brittle Because Ionic compounds are brittle because they have a crystal lattice structure. However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. In summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of. Ionic Compounds Brittle Because.
From slideplayer.com
Chemical Bonds. ppt download Ionic Compounds Brittle Because Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. See the study guide on the. Explain why most ionic compounds are strong and hard, yet brittle. In summary, brittleness is not a property uniquely associated with ionic compounds. When an external force. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Ionic Compounds Brittle Because In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are strong and hard because of the strong. See the study guide on the. It takes a large amount of mechanical force, such as striking a crystal. It. Ionic Compounds Brittle Because.
From slideplayer.com
Notes Ionic Bonds 1. Key Concept Ionic bonds form when electrons are Ionic Compounds Brittle Because When an external force is applied, it can shift the layers of ions, causing. Explain why most ionic compounds are strong and hard, yet brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are brittle due to. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Ionic Compounds Brittle Because Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are brittle because they have a crystal lattice structure. See the study guide on the. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic vs Molecular Compounds ppt download Ionic Compounds Brittle Because When an external force is applied, it can shift the layers of ions, causing. It takes a large amount of mechanical force, such as striking a crystal. See the study guide on the. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Ionic. Ionic Compounds Brittle Because.
From slideplayer.com
Materials Science Lesson ppt download Ionic Compounds Brittle Because It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical force, such as striking a crystal. However,. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation, free Ionic Compounds Brittle Because Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds are brittle because they have a crystal lattice structure. Explain why most ionic compounds are strong and hard, yet brittle. However, when that happens, it. Ionic Compounds Brittle Because.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Ionic Compounds Brittle Because Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the. Ionic Compounds Brittle Because.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Ionic Compounds Brittle Because Ionic compounds are strong and hard because of the strong. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle because they have a crystal lattice structure. However, when that happens, it brings ions of the same charge next. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Ionic Compounds Brittle Because Ionic compounds are strong and hard because of the strong. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. In summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of mechanical force, such as striking. Ionic Compounds Brittle Because.
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube Ionic Compounds Brittle Because It takes a large amount of mechanical force, such as striking a crystal. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard, but brittle.. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic Bond Chapter 5 Section ppt download Ionic Compounds Brittle Because Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. However, when that happens, it brings ions of the same charge next to each other (see below). Explain why most ionic compounds are strong and hard, yet brittle. It takes a large amount of mechanical force, such as striking a crystal. In. Ionic Compounds Brittle Because.
From slidetodoc.com
Chemistry Lesson 1 Ionic Compounds Ionic Bonds l Ionic Compounds Brittle Because However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are generally hard, but brittle. Ionic. Ionic Compounds Brittle Because.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Ionic Compounds Brittle Because However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard, but brittle. Explain why most ionic. Ionic Compounds Brittle Because.
From slideplayer.com
Lewis Dot Structures And… Ionic & Metallic Bonding. ppt download Ionic Compounds Brittle Because Ionic compounds are strong and hard because of the strong. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic Compounds Brittle Because.
From slideplayer.com
Ionic and Covalent Bonds ppt download Ionic Compounds Brittle Because Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. However, when that happens, it brings ions of the same charge next to each other (see below). It. Ionic Compounds Brittle Because.
From slideplayer.com
Notes Ionic Bonds 1. Key Concept Ionic bonds form when electrons are Ionic Compounds Brittle Because It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds are strong and hard because of the strong. When an external force is applied, it can shift the layers of ions, causing. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Ionic Compounds Brittle Because Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. However, when that happens, it brings ions of the same charge next to each other (see below). Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Explain why most ionic compounds. Ionic Compounds Brittle Because.
From slideplayer.com
Bonding and Properties ppt download Ionic Compounds Brittle Because See the study guide on the. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are strong and hard because of the. Ionic Compounds Brittle Because.
From slideplayer.com
Modern Chemistry Chapter 6 Chemical Bonding ppt download Ionic Compounds Brittle Because Ionic compounds are strong and hard because of the strong. Explain why most ionic compounds are strong and hard, yet brittle. When an external force is applied, it can shift the layers of ions, causing. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up. Ionic Compounds Brittle Because.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Ionic Compounds Brittle Because In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are brittle because they have a crystal lattice structure. See the study guide on the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical force, such as striking a crystal. Ionic Compounds Brittle Because.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Ionic Compounds Brittle Because Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. See the study guide on the. Ionic compounds are generally hard, but brittle. Explain why most ionic compounds are strong and hard, yet brittle. However, when that happens, it brings ions of the same charge next to each other (see below). It. Ionic Compounds Brittle Because.