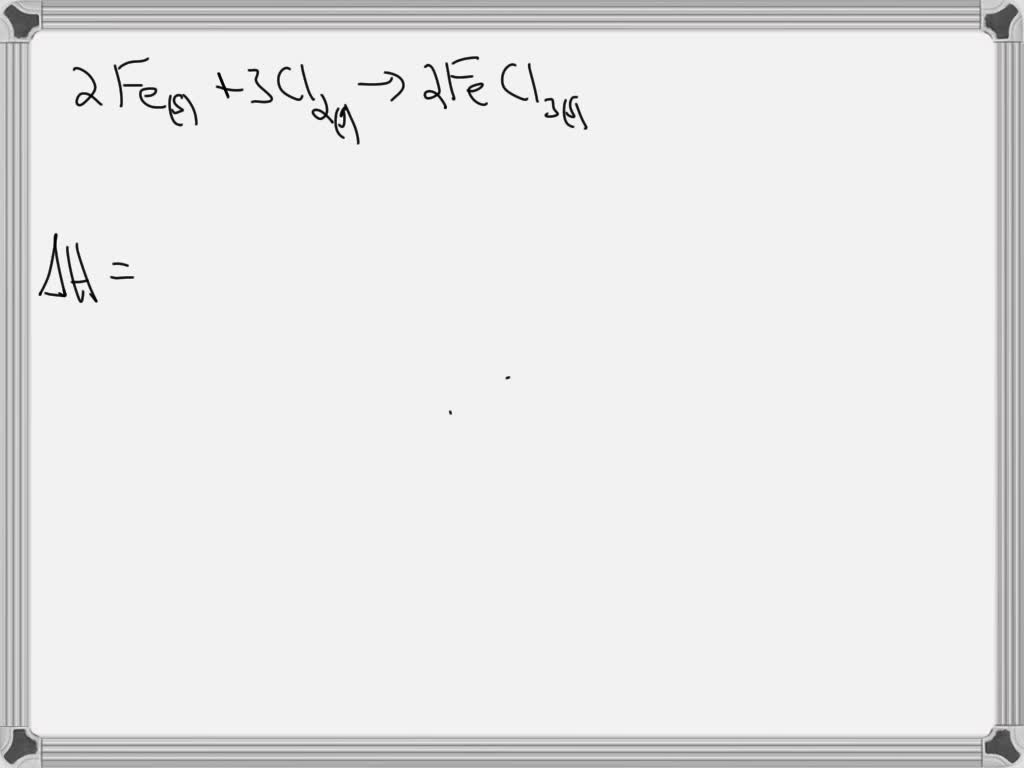Standard Enthalpy Of Formation Of H2O Equation . It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
from www.numerade.com
The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c.
SOLVED Calculate the standard enthalpy of formation and the standard
Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition.
From readingandwritingprojectcom.web.fc2.com
heat of formation for o2 Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of. Standard Enthalpy Of Formation Of H2O Equation.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Of H2O Equation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation Of H2O Equation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation of any element in its standard state is zero by definition. It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. The standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation Of H2O Equation.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation and the standard Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly. Standard Enthalpy Of Formation Of H2O Equation.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Of H2O Equation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Standard Enthalpy Of Formation Of H2O Equation.
From lessonschoolimbrowning.z14.web.core.windows.net
Heat Of Formation Equations Standard Enthalpy Of Formation Of H2O Equation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Of H2O Equation.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation Of H2O Equation.
From narodnatribuna.info
Enthalpy Equation Standard Enthalpy Of Formation Of H2O Equation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. It. Standard Enthalpy Of Formation Of H2O Equation.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Of Formation Of H2O Equation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. A standard. Standard Enthalpy Of Formation Of H2O Equation.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpy Of Formation Of H2O Equation It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist. Standard Enthalpy Of Formation Of H2O Equation.
From www.slideshare.net
Lect w10 abbrev_ thermochemistry_alg Standard Enthalpy Of Formation Of H2O Equation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard. Standard Enthalpy Of Formation Of H2O Equation.
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID6274598 Standard Enthalpy Of Formation Of H2O Equation It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of. Standard Enthalpy Of Formation Of H2O Equation.
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Of Formation Of H2O Equation.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Of H2O Equation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. It means that 393.509 kj of energy is released when one mole of. Standard Enthalpy Of Formation Of H2O Equation.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Enthalpy Of Formation Of H2O Equation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard state is zero by definition. It means that 393.509. Standard Enthalpy Of Formation Of H2O Equation.
From www.toppr.com
The standard enthalpy of formation of H2O (l) and Fe2O3 (s) are Standard Enthalpy Of Formation Of H2O Equation It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193. Standard Enthalpy Of Formation Of H2O Equation.
From byjus.com
21. At 300 K standard enthalpy of formation of C6H5COOH(s), CO2(g), and Standard Enthalpy Of Formation Of H2O Equation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. It means that 393.509 kj of energy is released when one mole of co 2 is formed from. Standard Enthalpy Of Formation Of H2O Equation.
From www.chegg.com
Solved Using The Standard Enthalpies Of Formation Listed Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Enthalpy Of Formation Of H2O Equation.
From www.numerade.com
SOLVED Using the standard enthalpies of formation, what is the Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation Of H2O Equation.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Of H2O Equation It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure and 25 ˚c. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is the enthalpy change when 1. Standard Enthalpy Of Formation Of H2O Equation.
From quizzlistreplevies.z13.web.core.windows.net
Heat Of Formation Equations Standard Enthalpy Of Formation Of H2O Equation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard state is zero by definition. It means that 393.509 kj of energy is released when one mole of co 2 is formed from. Standard Enthalpy Of Formation Of H2O Equation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. Standard. Standard Enthalpy Of Formation Of H2O Equation.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Of H2O Equation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation Of H2O Equation.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Enthalpy Of Formation Of H2O Equation.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.. Standard Enthalpy Of Formation Of H2O Equation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Of H2O Equation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. It means. Standard Enthalpy Of Formation Of H2O Equation.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Of Formation Of H2O Equation.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly. Standard Enthalpy Of Formation Of H2O Equation.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Of H2O Equation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The. Standard Enthalpy Of Formation Of H2O Equation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Of H2O Equation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation Of H2O Equation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Of H2O Equation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard Enthalpy Of Formation Of H2O Equation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Of H2O Equation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation Of H2O Equation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Of H2O Equation The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. 193. Standard Enthalpy Of Formation Of H2O Equation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Of H2O Equation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. The standard enthalpy of formation of any element in its standard state is zero by definition. It means that 393.509 kj of energy is released when one mole of co 2 is formed from graphite (c) and oxygen gas (o 2 ) at 1 atmospheric pressure. Standard Enthalpy Of Formation Of H2O Equation.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Of H2O Equation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. The standard. Standard Enthalpy Of Formation Of H2O Equation.