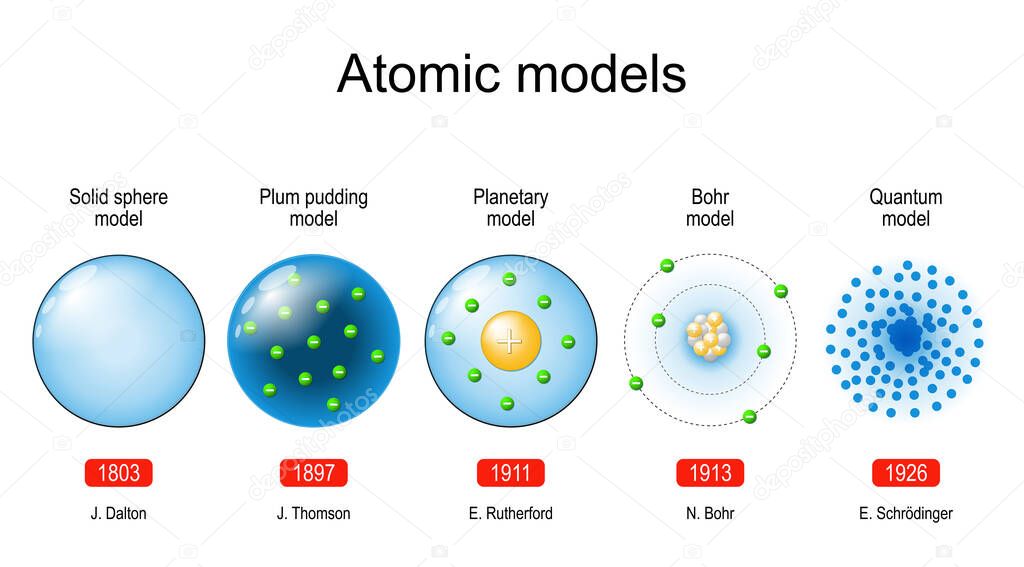
M And M Model Of An Atom . the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. The first three quantum numbers. N, l, m l, and m s. The principal quantum number (n), the orbital. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. m and m® model of the atom. an electron in an atom is completely described by four quantum numbers: this page contains materials for the session on the atomic models of rutherford and bohr. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. Use colored candy to represent subatomic particles and. learn about the bohr model of the atom.
from depositphotos.com
an electron in an atom is completely described by four quantum numbers: The first three quantum numbers. this page contains materials for the session on the atomic models of rutherford and bohr. The principal quantum number (n), the orbital. Use colored candy to represent subatomic particles and. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. learn about the bohr model of the atom. m and m® model of the atom.
Atom Modelleri Parçacıklar Hakkında Bilimsel Teori Fizik Vektör
M And M Model Of An Atom Use colored candy to represent subatomic particles and. m and m® model of the atom. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. this page contains materials for the session on the atomic models of rutherford and bohr. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. N, l, m l, and m s. The first three quantum numbers. Use colored candy to represent subatomic particles and. learn about the bohr model of the atom. The principal quantum number (n), the orbital. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. an electron in an atom is completely described by four quantum numbers:
From brainly.in
draw a diagram of bohrs model of an atom showing four energy shells M And M Model Of An Atom The principal quantum number (n), the orbital. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. this page contains materials for the session on the atomic models of rutherford and bohr. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum. M And M Model Of An Atom.
From topglobenews.com
A Brief History of the Atom Top Globe News M And M Model Of An Atom the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: an electron in an atom is completely described by four quantum numbers: Use colored candy to. M And M Model Of An Atom.
From www.shalom-education.com
Bohr's Model of the Atom GCSE Physics Revision M And M Model Of An Atom the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. learn about the bohr model of the atom. The first three quantum numbers. an electron in an atom is completely described by four quantum numbers: this page contains materials for the session on the atomic models. M And M Model Of An Atom.
From mavink.com
Types Of Atomic Models M And M Model Of An Atom the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. Use colored candy to represent subatomic particles and. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: learn about the bohr model of the atom.. M And M Model Of An Atom.
From scienceworldfactors.blogspot.com
Concepts of atom M And M Model Of An Atom m and m® model of the atom. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. an electron in an atom is. M And M Model Of An Atom.
From frecetovbpschematic.z4.web.core.windows.net
Diagram On An Atom M And M Model Of An Atom in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: this page contains materials for the session on the atomic models of rutherford and bohr. an electron in an atom is completely described by four quantum numbers: the bohr model of the atom was proposed by niels bohr. M And M Model Of An Atom.
From bgbasicscience.blogspot.com
Lets Get Inside An Atom!! The Science Station M And M Model Of An Atom the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. The first three quantum numbers. learn about the bohr model of the atom. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. See. M And M Model Of An Atom.
From www.shutterstock.com
Illustration Atomic Model Thomson Rutherford Bohr เวกเตอร์สต็อก (ปลอด M And M Model Of An Atom The first three quantum numbers. Use colored candy to represent subatomic particles and. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. The principal quantum number (n), the orbital. N, l, m l, and m s. the model explained how an atom absorb or emit radiation when electrons on. M And M Model Of An Atom.
From printablesacchellaxi.z4.web.core.windows.net
Structure Of Atom Bohr Model M And M Model Of An Atom Use colored candy to represent subatomic particles and. this page contains materials for the session on the atomic models of rutherford and bohr. The first three quantum numbers. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. See the main points of the model, how to calculate. M And M Model Of An Atom.
From ar.inspiredpencil.com
Atom Model Project M And M Model Of An Atom Use colored candy to represent subatomic particles and. this page contains materials for the session on the atomic models of rutherford and bohr. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. m and m® model of the atom. The first three quantum numbers. the. M And M Model Of An Atom.
From mungfali.com
Atomic Structure Of An Atom Model M And M Model Of An Atom The first three quantum numbers. m and m® model of the atom. Use colored candy to represent subatomic particles and. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. the model explained how an atom absorb or emit radiation when electrons on subatomic. M And M Model Of An Atom.
From www.hanlin.com
Edexcel A Level Physics复习笔记8.2 The Nuclear Model of the Atom翰林国际教育 M And M Model Of An Atom in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: The first three quantum numbers. this page contains materials for the session on the atomic models of rutherford and bohr. The principal quantum number (n), the orbital. the bohr model of the atom was proposed by niels bohr in. M And M Model Of An Atom.
From www.youtube.com
how to make a atom structure model 3d science project for science M And M Model Of An Atom learn about the bohr model of the atom. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: an electron in an atom is completely described by four quantum numbers: Use colored candy to represent subatomic particles and. N, l, m l, and m s. See the main points. M And M Model Of An Atom.
From www.youtube.com
Bohr's atomic model of nitrogen atom 3D atom model out of marbles M And M Model Of An Atom The first three quantum numbers. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. m and m® model of the atom. the bohr model of the atom. M And M Model Of An Atom.
From www.schoolsobservatory.org
Nuclear Physics The Schools' Observatory M And M Model Of An Atom in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. m and m® model of the atom. the bohr model of the atom was proposed by niels bohr. M And M Model Of An Atom.
From www.compoundchem.com
The History of the Atom Theories and Models Compound Interest M And M Model Of An Atom the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. this page contains materials for the session on the atomic models of rutherford and bohr. m and m® model of the atom. in atoms, an electrons location around the nucleus of an atom. M And M Model Of An Atom.
From circuitpennhockey01yh.z13.web.core.windows.net
Modern Atomic Model Diagram M And M Model Of An Atom The principal quantum number (n), the orbital. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: N, l, m l, and m s. Use colored candy to represent subatomic particles and. The first three quantum numbers. the bohr model of the atom was proposed by niels bohr in 1913. M And M Model Of An Atom.
From quizzfullnebesomux.z14.web.core.windows.net
Free Science Lessons History Of The Atom M And M Model Of An Atom The principal quantum number (n), the orbital. The first three quantum numbers. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: an electron in an atom is completely described by four quantum numbers: m and m® model of the atom. Use colored candy to represent subatomic particles and.. M And M Model Of An Atom.
From www.mindomo.com
Energy Mind Map M And M Model Of An Atom N, l, m l, and m s. Use colored candy to represent subatomic particles and. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. The principal. M And M Model Of An Atom.
From www.youtube.com
how to make 3d model of structure of atom using marble and cardboard M And M Model Of An Atom Use colored candy to represent subatomic particles and. N, l, m l, and m s. this page contains materials for the session on the atomic models of rutherford and bohr. an electron in an atom is completely described by four quantum numbers: See the main points of the model, how to calculate absorbed or emitted energy, and why. M And M Model Of An Atom.
From www.youtube.com
Atomic structure model project Atomic structure model 3d Atomic M And M Model Of An Atom the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: The first three quantum numbers. The principal quantum number (n), the orbital. m and m® model. M And M Model Of An Atom.
From www.youtube.com
Atom structure model atom project for school Atom project making M And M Model Of An Atom this page contains materials for the session on the atomic models of rutherford and bohr. m and m® model of the atom. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. The principal quantum number (n), the orbital. learn about the bohr model of the. M And M Model Of An Atom.
From circuitlibrarycorti.z13.web.core.windows.net
Atomic Structure Diagram Labelled M And M Model Of An Atom The first three quantum numbers. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. m and m® model of the atom. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. this page contains materials for the. M And M Model Of An Atom.
From www.vecteezy.com
Different models of atom vector illustration 21669330 Vector Art at M And M Model Of An Atom The principal quantum number (n), the orbital. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: Use colored candy to represent subatomic particles and. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. this page contains materials. M And M Model Of An Atom.
From www.savemyexams.com
Developing Models of the Atom Edexcel GCSE Physics Revision Notes 2018 M And M Model Of An Atom The first three quantum numbers. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. in atoms, an electrons location around the nucleus of an atom can be defined by four quantum numbers: this page contains materials for the session on the atomic models of rutherford and bohr. The. M And M Model Of An Atom.
From classnotes.org.in
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom M And M Model Of An Atom The principal quantum number (n), the orbital. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. an electron in an atom is completely described by four quantum numbers: The first three quantum numbers. this page contains materials for the session on the atomic models of rutherford and bohr.. M And M Model Of An Atom.
From in.pinterest.com
how to make a atom model science project Bohr atomic model atomic M And M Model Of An Atom The principal quantum number (n), the orbital. this page contains materials for the session on the atomic models of rutherford and bohr. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. N, l, m l, and m s. The first three quantum numbers. . M And M Model Of An Atom.
From repairmachineariel.z21.web.core.windows.net
Modeling The Structure Of An Atom M And M Model Of An Atom Use colored candy to represent subatomic particles and. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. this page contains materials for the session on the atomic models of rutherford and bohr. N, l, m l, and m s. an electron in an atom is completely. M And M Model Of An Atom.
From depositphotos.com
Atom Modelleri Parçacıklar Hakkında Bilimsel Teori Fizik Vektör M And M Model Of An Atom The principal quantum number (n), the orbital. The first three quantum numbers. learn about the bohr model of the atom. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. N, l, m l, and m s. the model explained how an atom absorb or emit radiation when electrons. M And M Model Of An Atom.
From howtofunda.com
3D Atom Model for School Science Exhibition Free Science Maths M And M Model Of An Atom the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. this page contains materials for the session on the atomic models of rutherford and. M And M Model Of An Atom.
From www.worksheetsplanet.com
Bohr's Atomic Model M And M Model Of An Atom m and m® model of the atom. The first three quantum numbers. learn about the bohr model of the atom. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. N, l, m l, and m s. The principal quantum number (n), the orbital. Use colored candy to represent. M And M Model Of An Atom.
From www.britannica.com
Bohr model Description & Development Britannica M And M Model Of An Atom an electron in an atom is completely described by four quantum numbers: See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. The principal quantum number (n), the orbital. N, l, m l, and m s. this page contains materials for the session on the atomic models of rutherford. M And M Model Of An Atom.
From mungfali.com
Atom Structure Diagram M And M Model Of An Atom N, l, m l, and m s. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. Use colored candy to represent subatomic particles and. learn about the bohr model of the atom. an electron in an atom is completely described by four quantum. M And M Model Of An Atom.
From ar.inspiredpencil.com
Bohr Atomic Theory M And M Model Of An Atom N, l, m l, and m s. The principal quantum number (n), the orbital. the model explained how an atom absorb or emit radiation when electrons on subatomic level jump between the allowed and. Use colored candy to represent subatomic particles and. m and m® model of the atom. an electron in an atom is completely described. M And M Model Of An Atom.
From tolfsbook.weebly.com
tolfsbook Blog M And M Model Of An Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. the bohr model of the atom was proposed by niels bohr in 1913 as an expansion on and correction of the rutherford model. The principal quantum number (n), the orbital. the model explained how an atom absorb or emit. M And M Model Of An Atom.