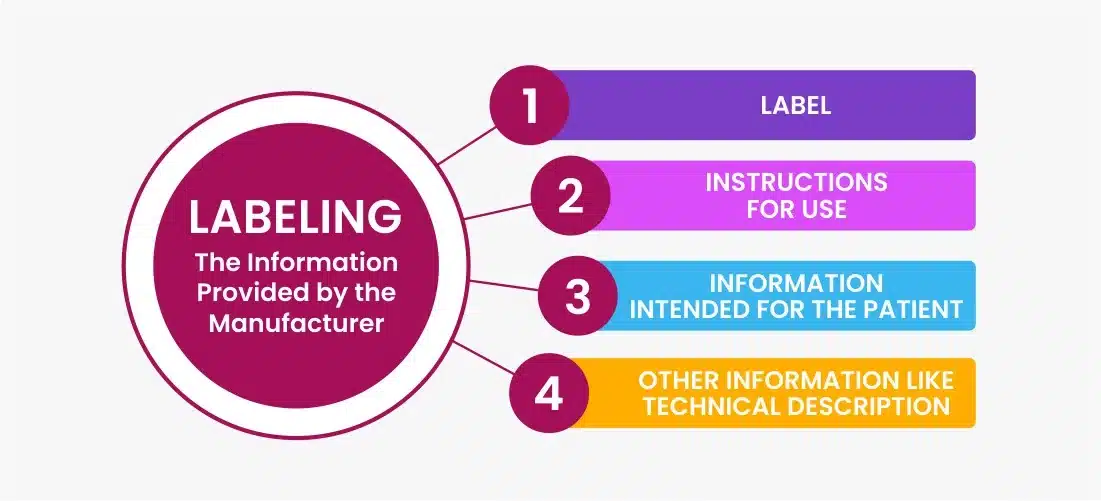
Medical Device Labeling Procedure . The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member.
from medicaldevicelicense.com
•understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member.
Medical Device Blog I Discover Latest Expert Analysis of MDR
Medical Device Labeling Procedure medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively.
From www.presentationeze.com
FDA Medical Device Labeling requirements. PresentationEZE Medical Device Labeling Procedure The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. labeling and packaging is key to ensure that a medical device or in vitro diagnostic. Medical Device Labeling Procedure.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Medical Device Labeling Procedure this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. labeling and packaging is key to ensure that a medical device or in vitro diagnostic. Medical Device Labeling Procedure.
From blogs.sw.siemens.com
Siemens PLM for Medical Devices Labeling and UDI solution Medical Device Labeling Procedure medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. principles of labelling for medical devices and. Medical Device Labeling Procedure.
From issuu.com
Medical Device Labeling Requirements VISTAAR by VISTAAR Issuu Medical Device Labeling Procedure principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. labeling and packaging is key to ensure that a medical device or in. Medical Device Labeling Procedure.
From bizmanualz.com
Labeling Procedure Medical Device Labeling Procedure The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different types. Medical Device Labeling Procedure.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Procedure principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu. Medical Device Labeling Procedure.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Medical Device Labeling Procedure medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. principles of labelling for medical devices and. Medical Device Labeling Procedure.
From dxolizkya.blob.core.windows.net
Medical Device Labelling Requirements at William Smith blog Medical Device Labeling Procedure principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as. Medical Device Labeling Procedure.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Labeling Procedure principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us. Medical Device Labeling Procedure.
From medicaldeviceacademy.com
Labeling process flowchart Medical Device Academy Medical Device Labeling Procedure this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. labeling and packaging is key to ensure that a medical device or in. Medical Device Labeling Procedure.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Labeling Procedure this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. •understand the requirements for medical device labeling •review some key. Medical Device Labeling Procedure.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Labeling Procedure labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. •understand the requirements for. Medical Device Labeling Procedure.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Labeling Procedure •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. principles. Medical Device Labeling Procedure.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Procedure this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. medical device safely should be provided on the medical device itself, and/or on primary packing. Medical Device Labeling Procedure.
From aplyonqms.com
Medical Device Labeling Procedure Bundle A. P. LYON Medical Device Labeling Procedure •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. The purpose of. Medical Device Labeling Procedure.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Labeling Procedure •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. labeling and packaging is key to ensure. Medical Device Labeling Procedure.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Labeling Procedure medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. this post will discuss what counts as a medical device label, where they are. Medical Device Labeling Procedure.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labeling Procedure labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical. Medical Device Labeling Procedure.
From medicaldevicelicense.com
Medical Device Blog I Discover Latest Expert Analysis of MDR Medical Device Labeling Procedure labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to. Medical Device Labeling Procedure.
From aplyonqms.com
Medical Device Labeling Procedure Bundle A. P. LYON Medical Device Labeling Procedure The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different types. Medical Device Labeling Procedure.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Procedure medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. •understand. Medical Device Labeling Procedure.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Labeling Procedure labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to. Medical Device Labeling Procedure.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Procedure medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member.. Medical Device Labeling Procedure.
From hxejjejdf.blob.core.windows.net
Medical Device Labeling Sop at Eldon Black blog Medical Device Labeling Procedure labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. medical device safely should. Medical Device Labeling Procedure.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Labeling Procedure •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. The purpose of. Medical Device Labeling Procedure.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Medical Device Labeling Procedure principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. medical device safely should be provided on the medical device itself, and/or on. Medical Device Labeling Procedure.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Medical Device Labeling Procedure •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. labeling and packaging is key to ensure that a medical device or in. Medical Device Labeling Procedure.
From issuu.com
EU MDR Medical Device Labeling changes and challenges by martinafrotz Medical Device Labeling Procedure this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. labeling and packaging is key to ensure that a medical device or in. Medical Device Labeling Procedure.
From hxejjejdf.blob.core.windows.net
Medical Device Labeling Sop at Eldon Black blog Medical Device Labeling Procedure principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. this post will discuss what counts as a medical device label, where they are required, and look at the key. Medical Device Labeling Procedure.
From www.shutterstock.com
3,317 Medical Device Labeling Images, Stock Photos & Vectors Shutterstock Medical Device Labeling Procedure labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key. Medical Device Labeling Procedure.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Medical Device Labeling Procedure labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key. Medical Device Labeling Procedure.
From www.freseniusmedicalcare.pl
Rozporządzenie w sprawie wyrobów medycznych Fresenius Medical Care Medical Device Labeling Procedure The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. principles of labelling for. Medical Device Labeling Procedure.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Procedure this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. •understand the requirements for medical device labeling. Medical Device Labeling Procedure.
From aplyonqms.com
Medical Device Labeling Procedure Bundle A. P. LYON Medical Device Labeling Procedure medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. this post will discuss what counts as a medical device label, where they are required, and look. Medical Device Labeling Procedure.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Medical Device Labeling Procedure principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. medical device safely should be provided on the medical device itself, and/or on primary packing when it is difficult to place a the. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. •understand. Medical Device Labeling Procedure.