Titration Explanation . Titration is a common laboratory method of using quantitative chemical analysis. Analysis of soil samples by titration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. This method is used to determine the unidentified. The known solution (titrate) is added in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the.
from www.chegg.com
Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Analysis of soil samples by titration. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration is a common laboratory method of using quantitative chemical analysis. The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. This method is used to determine the unidentified.
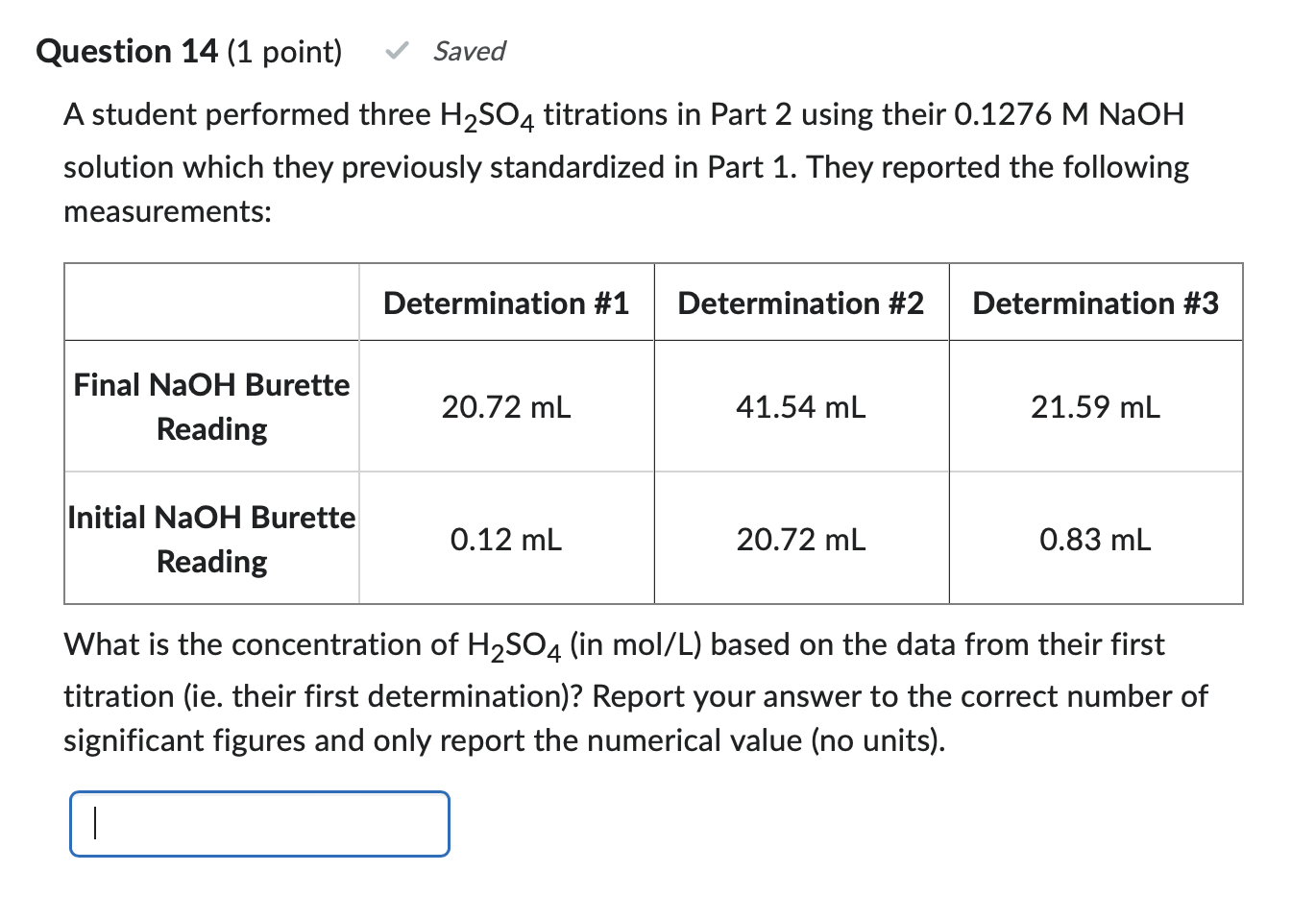
Solved A student performed three H2SO4 titrations in Part 2
Titration Explanation A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. This method is used to determine the unidentified. The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Analysis of soil samples by titration. Titration is a common laboratory method of using quantitative chemical analysis. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Explanation The known solution (titrate) is added in. Titration is a common laboratory method of using quantitative chemical analysis. This method is used to determine the unidentified. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A typical titration begins with a beaker or erlenmeyer flask. Titration Explanation.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Explanation A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Analysis of soil samples by titration. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. The known solution (titrate) is added in. This method is used to determine the unidentified.. Titration Explanation.
From www.studocu.com
Titration Report BTEC Applied Science Unit2 A Titration Report Titration Explanation A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. A titration is a technique where a solution of known concentration is used to determine the. Titration Explanation.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Explanation This method is used to determine the unidentified. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration. Titration Explanation.
From www.vrogue.co
Precipitation Titration Curves vrogue.co Titration Explanation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Analysis of soil samples by titration. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Titration, process of chemical analysis in which the quantity of some constituent. Titration Explanation.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Explanation The known solution (titrate) is added in. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. This method is used to determine the unidentified. Titration is a common laboratory method of using quantitative chemical analysis. Titration is a technique of determining the concentration. Titration Explanation.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Explanation Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. This method is used to determine the unidentified. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Analysis of soil samples by titration.. Titration Explanation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Explanation Analysis of soil samples by titration. The known solution (titrate) is added in. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Titration is a common laboratory method of using quantitative chemical analysis. A titration is a technique where a solution of known concentration is used to determine the concentration of. Titration Explanation.
From www.chegg.com
Solved A student performed three aqueous layer titrations in Titration Explanation A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Analysis of soil samples by titration. The known solution (titrate) is added in. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration Explanation.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Explanation Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. A typical titration begins with a beaker or erlenmeyer flask containing a very precise. Titration Explanation.
From www.chegg.com
Solved A student performed three H2SO4 titrations in Part 2 Titration Explanation Analysis of soil samples by titration. Titration is a common laboratory method of using quantitative chemical analysis. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. This method is used. Titration Explanation.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Explanation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration, process of chemical analysis in which the quantity of. Titration Explanation.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Explanation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Analysis of soil samples by titration. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration involves the gradual. Titration Explanation.
From www.cloudizsexy.com
Acid Base Titration Titration Curves Equivalence Point And Indicators Titration Explanation Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Analysis of soil samples by titration. Titration is a common laboratory method of using quantitative chemical analysis. Titration involves the gradual addition of a reagent of known concentration, known as the titrant,. Titration Explanation.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Explanation This method is used to determine the unidentified. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a common laboratory method of using quantitative chemical analysis. Analysis of. Titration Explanation.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Explanation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. This method is used to determine the unidentified. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly. Titration Explanation.
From www.youtube.com
Conductometric titration (Precipitation titration of KCl vs AgNO3 and Titration Explanation This method is used to determine the unidentified. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is used to determine the concentration of an. Titration Explanation.
From slideplayer.com
Big Idea 1 Properties of Matter Part II. ppt download Titration Explanation Analysis of soil samples by titration. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a common laboratory method of using quantitative chemical analysis. This method is used. Titration Explanation.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Explanation A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. This method is used to determine the unidentified. The known solution. Titration Explanation.
From www.youtube.com
Back Titration Example YouTube Titration Explanation The known solution (titrate) is added in. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. This method is used to determine the unidentified. A titration is a technique where a solution of known concentration is used to determine the concentration of. Titration Explanation.
From www.chegg.com
Solved 10. Explain why the pH titration curve shown below Titration Explanation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Analysis of soil samples by titration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The known solution. Titration Explanation.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Explanation The known solution (titrate) is added in. This method is used to determine the unidentified. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Analysis of soil samples by titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Explanation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Explanation The known solution (titrate) is added in. Titration is a common laboratory method of using quantitative chemical analysis. Analysis of soil samples by titration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A typical titration begins with a beaker or erlenmeyer. Titration Explanation.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Explanation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This method is used to determine the unidentified. Titration,. Titration Explanation.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Explanation A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. This method is used to determine the unidentified. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Analysis of. Titration Explanation.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Explanation Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. This method is used to determine the unidentified. A typical titration begins with a. Titration Explanation.
From www.adda247.com
Titration Formula Explanation, Types, Calculation, Procedure, Practice Titration Explanation Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration involves the gradual addition of. Titration Explanation.
From www.studypool.com
SOLUTION Precipitation titration method , types, Explanation and Titration Explanation The known solution (titrate) is added in. This method is used to determine the unidentified. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. A titration is a technique where a solution of known concentration is used to determine the concentration of an. Titration Explanation.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration Explanation Titration is a common laboratory method of using quantitative chemical analysis. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample. Titration Explanation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Explanation A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. This method is used to determine the unidentified. The known solution (titrate) is added in. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration Explanation.
From www.youtube.com
Titration How to perform Titration Experiment/Practical YouTube Titration Explanation Analysis of soil samples by titration. Titration is a common laboratory method of using quantitative chemical analysis. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly. Titration Explanation.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Titration Explanation Analysis of soil samples by titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is. Titration Explanation.
From mavink.com
Titration Labeled Titration Explanation The known solution (titrate) is added in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. This method is used to determine the unidentified. Titration, process of. Titration Explanation.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Titration Explanation A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one. Titration Explanation.
From mungfali.com
Acid Base Titration Procedure Titration Explanation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a common laboratory method of using quantitative chemical analysis. A typical titration begins with a beaker or erlenmeyer flask containing a very precise amount of the analyte and a small amount. Titration Explanation.