Turbidity Meaning In Lucas Test . The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. Conversely, secondary alcohols produce reasonably stable 2 o. Takes longer time to turn turbid than tertiary alcohols i.e. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Primary secondary and tertiary alcohols react with. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide.
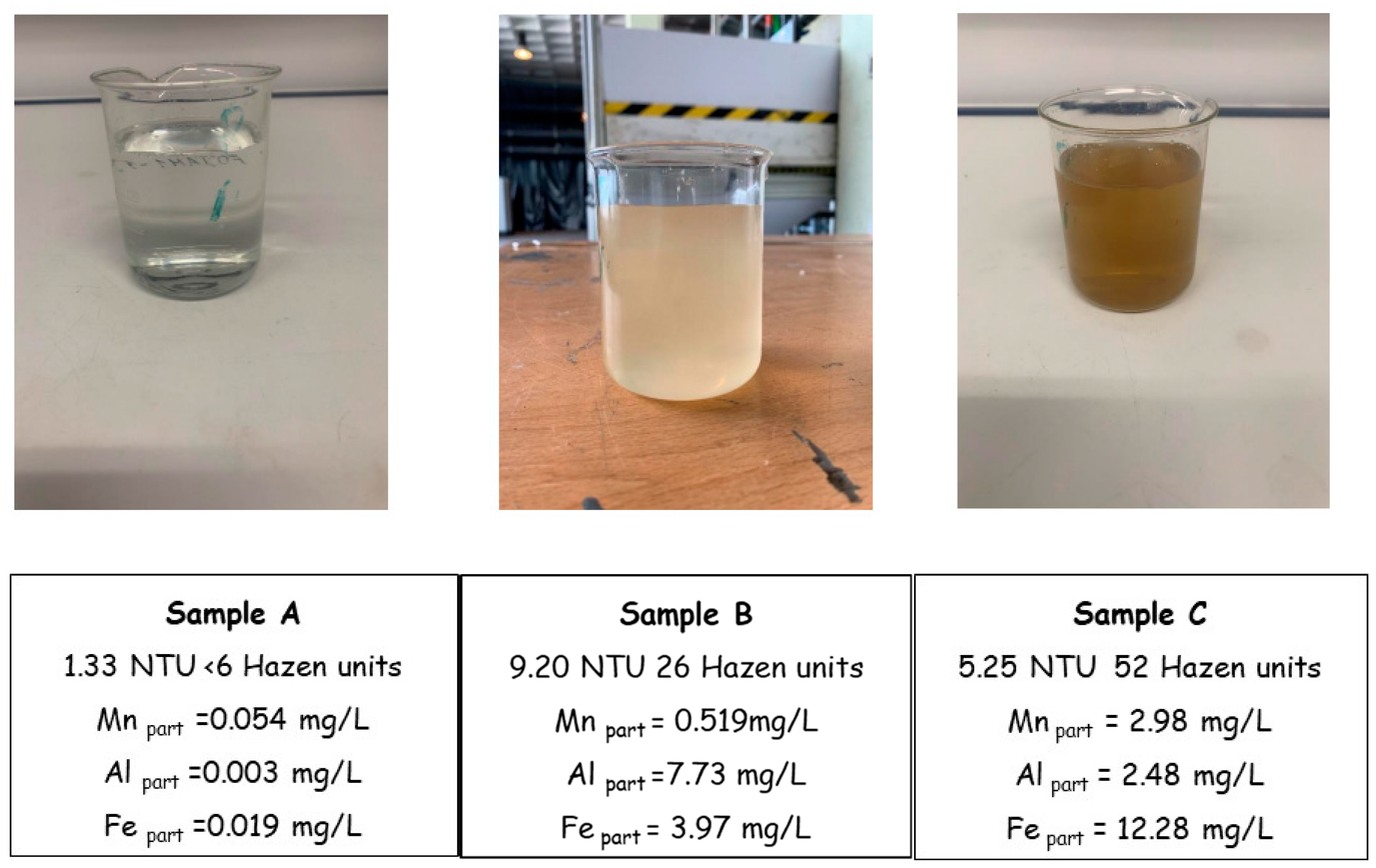
from www.mdpi.com
The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Takes longer time to turn turbid than tertiary alcohols i.e. Conversely, secondary alcohols produce reasonably stable 2 o. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Primary secondary and tertiary alcohols react with.
Water Free FullText Physicochemical Parameters in the Generation
Turbidity Meaning In Lucas Test There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Primary secondary and tertiary alcohols react with. The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. Takes longer time to turn turbid than tertiary alcohols i.e. Conversely, secondary alcohols produce reasonably stable 2 o. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide.
From civiljungle.com
All About Turbidity of Water What Is Turbidity of Water Procedure Turbidity Meaning In Lucas Test In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Conversely, secondary alcohols produce reasonably stable 2 o. Takes longer time to turn turbid than tertiary alcohols i.e. Lucas test is based on the difference in reactivity of alcohols with hydrogen. Turbidity Meaning In Lucas Test.
From tardigrade.in
X gives white turbidity with Lucas reagent instantly. X and Y both turn Turbidity Meaning In Lucas Test Primary secondary and tertiary alcohols react with. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. There is immediate turbidity in tertiary alcohols as a result. Turbidity Meaning In Lucas Test.
From www.youtube.com
Lucas Probe (Lucas test) YouTube Turbidity Meaning In Lucas Test The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. The lucas test, also known as the lucas reagent. Turbidity Meaning In Lucas Test.
From www.therma.com
How is Turbidity Measured? Therma Turbidity Meaning In Lucas Test Takes longer time to turn turbid than tertiary alcohols i.e. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. The lucas test, also known as the lucas reagent test, is a chemical test. Turbidity Meaning In Lucas Test.
From chemistnotes.com
McFarland Turbidity Test Easy introduction, protocols Chemistry Notes Turbidity Meaning In Lucas Test The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. Conversely, secondary alcohols produce reasonably stable 2 o. Takes longer time to turn turbid than tertiary alcohols i.e. The lucas test, also known as. Turbidity Meaning In Lucas Test.
From aurigaresearch.com
Turbidity Test of Drinking water Turbidity Meaning In Lucas Test The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Primary secondary and tertiary alcohols react with. The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. There is immediate turbidity in. Turbidity Meaning In Lucas Test.
From testbook.com
Lucas Test Definition, Examples, Test for Alcohols, Mechanism Turbidity Meaning In Lucas Test There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. Takes longer time to turn turbid than tertiary alcohols i.e. Conversely, secondary alcohols produce reasonably stable 2 o. The reactivity of the alcohol with. Turbidity Meaning In Lucas Test.
From waterfilterguru.com
What Is Turbidity in Water? (A Water Doctor Explains) Turbidity Meaning In Lucas Test The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Primary secondary and tertiary alcohols react with. The reactivity of the alcohol. Turbidity Meaning In Lucas Test.
From www.lovibond.com
Turbidity Measurement in Pool Water Lovibond Water Testing and Colour Turbidity Meaning In Lucas Test There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Takes longer time to turn turbid than tertiary alcohols i.e. Conversely, secondary alcohols produce reasonably stable 2 o. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. Primary secondary and tertiary alcohols react. Turbidity Meaning In Lucas Test.
From mavink.com
Water Turbidity Color Chart Turbidity Meaning In Lucas Test In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Takes longer time to turn turbid than tertiary alcohols i.e. Primary secondary and tertiary alcohols react with. The reactivity of the alcohol with lucas reagent is measured by the degree of. Turbidity Meaning In Lucas Test.
From www.youtube.com
Turbidity Water Quality Testing Activity YouTube Turbidity Meaning In Lucas Test Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Takes longer time to turn turbid than tertiary alcohols i.e. In addition to the lucas reagent in the solution containing. Turbidity Meaning In Lucas Test.
From www.researchgate.net
Turbidity values at distinct periods after treatments a. 1 h; b. 6 h Turbidity Meaning In Lucas Test The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. There is immediate turbidity in tertiary alcohols as a result of the. Turbidity Meaning In Lucas Test.
From netsolwater.com
How to determine turbidity of drinking water Turbidity Meaning In Lucas Test In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. Primary secondary and tertiary alcohols react with. Lucas test. Turbidity Meaning In Lucas Test.
From chemicalnote.com
Distinction of Alcohols Victor Meyer’s method and Lucas test. Turbidity Meaning In Lucas Test There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Conversely, secondary alcohols produce reasonably stable 2 o. Lucas test is based. Turbidity Meaning In Lucas Test.
From www.toppr.com
Which of the following alcohols give immediate turbidity with Lucas Turbidity Meaning In Lucas Test Conversely, secondary alcohols produce reasonably stable 2 o. Primary secondary and tertiary alcohols react with. Takes longer time to turn turbid than tertiary alcohols i.e. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. The lucas test, also known as the lucas reagent test, is a chemical. Turbidity Meaning In Lucas Test.
From studiousguy.com
Turbidity Definition, Causes, Measurement, and Examples StudiousGuy Turbidity Meaning In Lucas Test The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Takes longer time to turn turbid than tertiary alcohols. Turbidity Meaning In Lucas Test.
From shriramlab.org
Turbidity in Drinking Water Importance, Measurement, and Impact Turbidity Meaning In Lucas Test Primary secondary and tertiary alcohols react with. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in. Turbidity Meaning In Lucas Test.
From www.slideserve.com
PPT URINALYSIS PowerPoint Presentation, free download ID5640119 Turbidity Meaning In Lucas Test Takes longer time to turn turbid than tertiary alcohols i.e. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Primary secondary and tertiary alcohols react with. Conversely, secondary alcohols produce reasonably stable 2 o. The lucas test, also known as. Turbidity Meaning In Lucas Test.
From www.youtube.com
Which of the following can give immediate turbidity on treatment with Turbidity Meaning In Lucas Test Primary secondary and tertiary alcohols react with. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Lucas test. Turbidity Meaning In Lucas Test.
From puresoluble.com
Microtubule Turbidity Assay Turbidity Meaning In Lucas Test The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Primary secondary and tertiary alcohols react with. Conversely, secondary alcohols produce reasonably. Turbidity Meaning In Lucas Test.
From www.youtube.com
Lucas test Practical and mechanism explained YouTube Turbidity Meaning In Lucas Test In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Primary secondary and tertiary alcohols react with. Conversely, secondary alcohols produce reasonably stable 2 o. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. Takes longer. Turbidity Meaning In Lucas Test.
From www.slideserve.com
PPT Water Quality Parameters PowerPoint Presentation, free download Turbidity Meaning In Lucas Test In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Primary secondary and tertiary alcohols react with. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Conversely, secondary. Turbidity Meaning In Lucas Test.
From studiousguy.com
Turbidity Definition, Causes, Measurement, and Examples StudiousGuy Turbidity Meaning In Lucas Test Takes longer time to turn turbid than tertiary alcohols i.e. The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. In addition. Turbidity Meaning In Lucas Test.
From www.vrogue.co
Turbidity Definition Causes Measurement And Examples vrogue.co Turbidity Meaning In Lucas Test The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Takes longer time to turn turbid than tertiary alcohols i.e. Primary secondary and tertiary alcohols react. Turbidity Meaning In Lucas Test.
From www.youtube.com
Turbidity test YouTube Turbidity Meaning In Lucas Test Takes longer time to turn turbid than tertiary alcohols i.e. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity. Turbidity Meaning In Lucas Test.
From www.mdpi.com
Water Free FullText Physicochemical Parameters in the Generation Turbidity Meaning In Lucas Test The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Conversely, secondary alcohols produce reasonably stable 2 o. Primary secondary and tertiary alcohols react with. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. Takes longer time to turn. Turbidity Meaning In Lucas Test.
From pubs.rsc.org
Water turbidity sensing using a smartphone RSC Advances (RSC Turbidity Meaning In Lucas Test In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution. Conversely, secondary alcohols produce reasonably stable 2 o. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. Primary secondary and tertiary alcohols react with. There is. Turbidity Meaning In Lucas Test.
From www.renkeer.com
What Is a Turbidity Sensor? How to Choose? Renke Turbidity Meaning In Lucas Test Conversely, secondary alcohols produce reasonably stable 2 o. The reactivity of the alcohol with lucas reagent is measured by the degree of turbidity which may vary from colorless to turbid. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. Takes longer time to turn turbid than tertiary alcohols i.e. There is immediate turbidity in tertiary. Turbidity Meaning In Lucas Test.
From www.numerade.com
SOLVEDIn Lucas test, the correct statement is / are (A) Alcohols are Turbidity Meaning In Lucas Test Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to. Turbidity Meaning In Lucas Test.
From kimiagar2010.blogspot.com
Detachment and indentify of organic compound Lucas' reagent , MSDS Turbidity Meaning In Lucas Test Primary secondary and tertiary alcohols react with. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. Takes longer time to turn turbid than tertiary alcohols i.e. Conversely, secondary alcohols produce reasonably stable 2 o. In addition to the lucas reagent in the solution. Turbidity Meaning In Lucas Test.
From www.toppr.com
Which alcohol gives immediate turbidity with Lucas reagent? Turbidity Meaning In Lucas Test There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary (3°) alcohols. In addition to the lucas reagent in the solution containing secondary alcohol,. Turbidity Meaning In Lucas Test.
From studiousguy.com
Turbidity Definition, Causes, Measurement, and Examples StudiousGuy Turbidity Meaning In Lucas Test There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Conversely, secondary alcohols produce reasonably stable 2 o. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is. Turbidity Meaning In Lucas Test.
From www.slideserve.com
PPT URINALYSIS PowerPoint Presentation, free download ID2240987 Turbidity Meaning In Lucas Test There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. The lucas test, also known as the lucas reagent test, is a chemical test employed to differentiate between primary (1°), secondary (2°), and tertiary. Turbidity Meaning In Lucas Test.
From www.youtube.com
Chemical Tests for Alcohols Lucas Test & Oxidation Tests // HSC Turbidity Meaning In Lucas Test Takes longer time to turn turbid than tertiary alcohols i.e. Primary secondary and tertiary alcohols react with. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. In addition to the lucas reagent in the solution containing secondary alcohol, an oily layer is formed in three to five minutes and turbidity is observed in the solution.. Turbidity Meaning In Lucas Test.
From www.slideserve.com
PPT Urine analysis PowerPoint Presentation, free download ID8706530 Turbidity Meaning In Lucas Test Takes longer time to turn turbid than tertiary alcohols i.e. There is immediate turbidity in tertiary alcohols as a result of the lucas reagent's formation of an extremely stable 3 o cation. Primary secondary and tertiary alcohols react with. Lucas test is based on the difference in reactivity of alcohols with hydrogen halide. The lucas test, also known as the. Turbidity Meaning In Lucas Test.