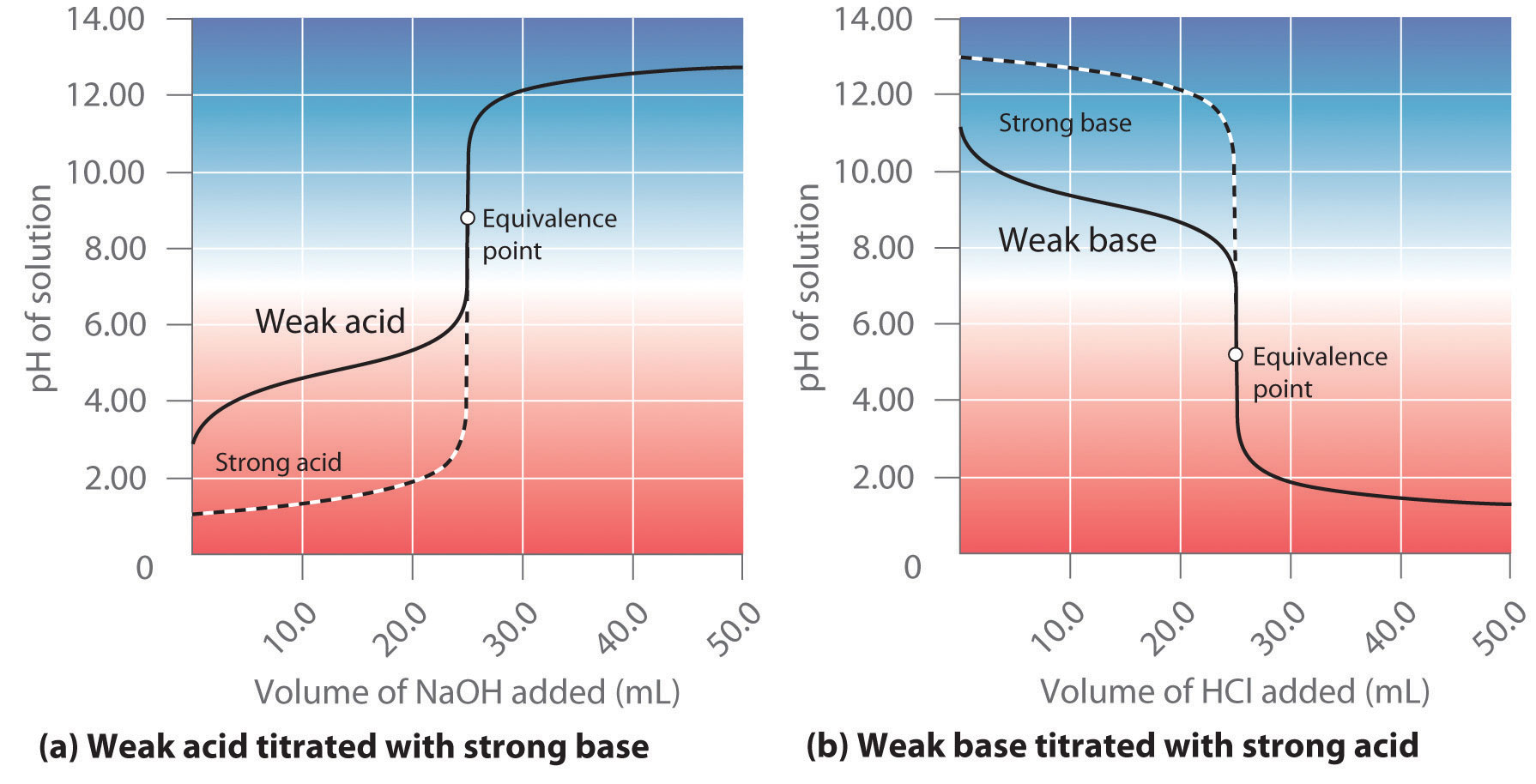
Titration At Equivalence Point . When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. Sorting out some confusing terms. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Although the initial volume and. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. Equivalence point (v = 25 ml): Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong.
from chem.libretexts.org
Although the initial volume and. Equivalence point (v = 25 ml): The equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sorting out some confusing terms. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph.
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts
Titration At Equivalence Point The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. Equivalence point (v = 25 ml): Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. Although the initial volume and. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration At Equivalence Point The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. The equivalence point of a chemical. Titration At Equivalence Point.
From ar.inspiredpencil.com
H3po4 Titration Curve Titration At Equivalence Point A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. The equivalence point of a titration. Although the. Titration At Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration At Equivalence Point A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. Equivalence point (v. Titration At Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration At Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. At the equivalence point in. Titration At Equivalence Point.
From mungfali.com
Equivalence Points On Titration Graph Titration At Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point),. Titration At Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration At Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Although the initial volume and. Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. A drastic rise in ph is observed. Titration At Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration At Equivalence Point A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Sorting out some confusing terms. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. Sketch out a plot. Titration At Equivalence Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration At Equivalence Point Sorting out some confusing terms. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong.. Titration At Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration At Equivalence Point Although the initial volume and. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. Titration At Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Titration At Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sorting out some confusing terms. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph.. Titration At Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration At Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration At Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration At Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Equivalence point (v = 25 ml): At the equivalence point. Titration At Equivalence Point.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Titration At Equivalence Point Although the initial volume and. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sorting out some confusing terms. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sketch. Titration At Equivalence Point.
From schoolbag.info
ACIDBASE TITRATIONS ADDITIONAL ASPECTS OF AQUEOUS EQUILIBRIA Titration At Equivalence Point A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. When 25 ml. Titration At Equivalence Point.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube Titration At Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence point (v = 25 ml): The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid). Titration At Equivalence Point.
From www.youtube.com
Titrations and the M1V1=M2V2 Math at The Equivalence Point YouTube Titration At Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence point (v = 25 ml): A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. The titration curve for the titration of 25.00 ml. Titration At Equivalence Point.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration At Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sorting out some confusing terms. Equivalence point (v = 25 ml): The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. When 25 ml of titrant has been added (the equivalence point), the. Titration At Equivalence Point.
From www.writework.com
Titration of amino acids WriteWork Titration At Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sorting out some confusing terms. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. Titration At Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Titration At Equivalence Point Equivalence point (v = 25 ml): A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Sorting out some confusing terms. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the equivalence. Titration At Equivalence Point.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration At Equivalence Point The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. Sorting out some confusing terms. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has. Titration At Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration At Equivalence Point Sorting out some confusing terms. Although the initial volume and. The equivalence point of a titration. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow.. Titration At Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration At Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. Sorting out some confusing terms. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. Titration At Equivalence Point.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration At Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sorting out some confusing terms. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. At the equivalence point in a neutralization, the moles of acid. Titration At Equivalence Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration At Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. When 25 ml of titrant has been added (the equivalence point), the ph is well above. Titration At Equivalence Point.
From saylordotorg.github.io
AcidBase Titrations Titration At Equivalence Point Equivalence point (v = 25 ml): The titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. Sorting out some confusing terms. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph. Titration At Equivalence Point.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration At Equivalence Point Sorting out some confusing terms. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Equivalence point (v = 25 ml): When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. A drastic rise in ph. Titration At Equivalence Point.
From www.vrogue.co
Point In Acid Base Titration The Equivalence Point So vrogue.co Titration At Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. Sorting out some confusing terms. A drastic rise in ph. Titration At Equivalence Point.
From www.coursehero.com
[Solved] Calculate the pH at the equivalence point for the titration Titration At Equivalence Point Sorting out some confusing terms. Although the initial volume and. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a chemical reaction. Titration At Equivalence Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Titration At Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. The equivalence point of a. Titration At Equivalence Point.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there Titration At Equivalence Point A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Although the initial volume and. Sketch out a plot representing the titration of a. Titration At Equivalence Point.
From www.numerade.com
SOLVED Calculate the pH at the equivalence point for the titration of Titration At Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. The titration curve. Titration At Equivalence Point.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration At Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. Sorting out some confusing terms. Although the initial volume and.. Titration At Equivalence Point.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration At Equivalence Point The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. When 25 ml of titrant has been added (the equivalence point),. Titration At Equivalence Point.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration At Equivalence Point The equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence point (v = 25 ml): When 25 ml. Titration At Equivalence Point.
From mavink.com
What Is The Equivalence Point Of A Titration Titration At Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. Equivalence point (v = 25 ml): The titration curve for the titration of 25.00 ml of 0.100. Titration At Equivalence Point.