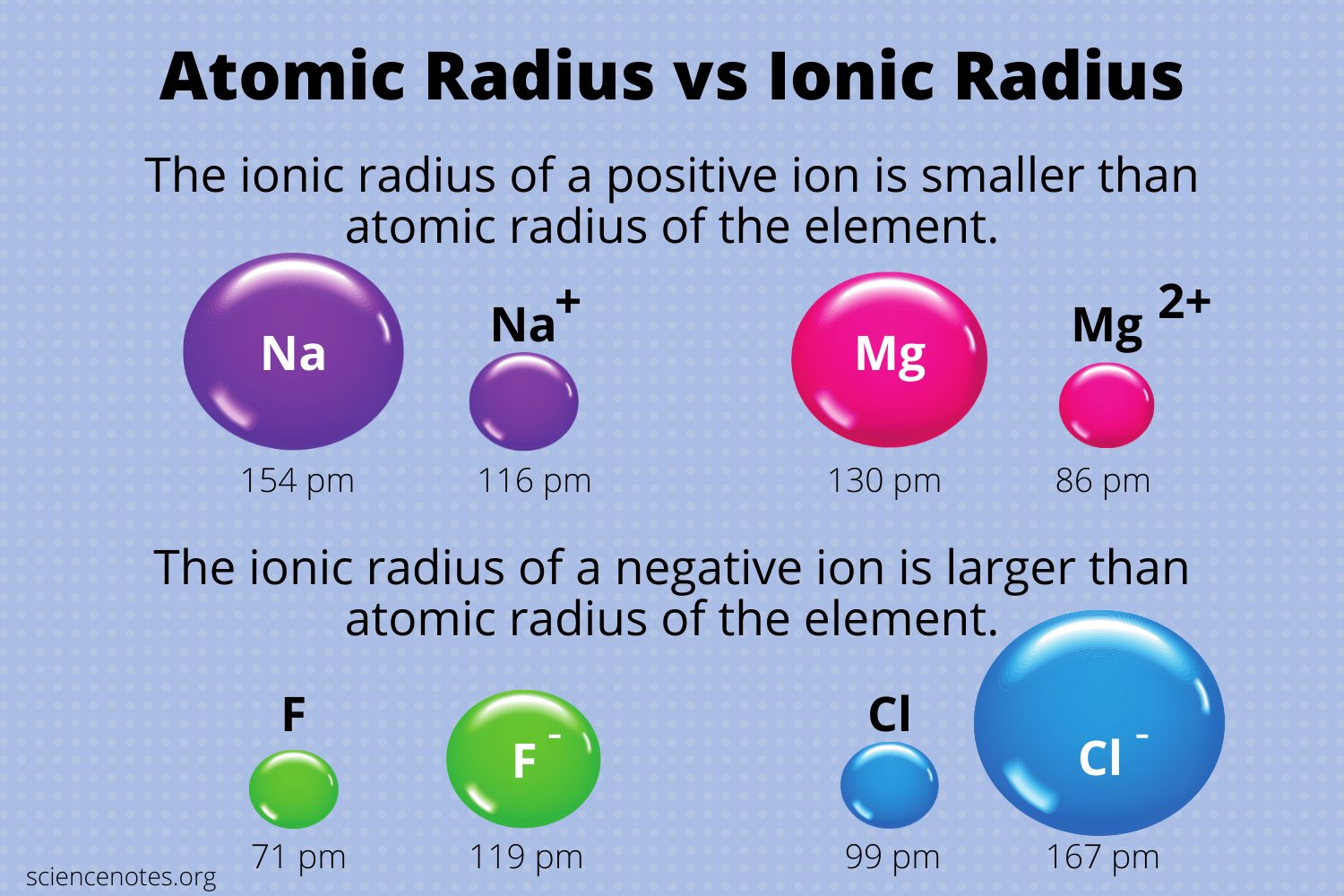
Magnesium Ions Radii . atomic and ionic radius. Typical values range from 30åpm (0.3åä) to over 200åpm. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. Although neither atoms nor ions have. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. Radii of atoms and ions. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. This page explains the various measures of atomic radius, and then looks at the way it varies.
from inspiritvr.com
a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. This page explains the various measures of atomic radius, and then looks at the way it varies. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. Although neither atoms nor ions have. atomic and ionic radius. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. Radii of atoms and ions. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Typical values range from 30åpm (0.3åä) to over 200åpm.
Ionic radii Study Guide Inspirit Learning Inc
Magnesium Ions Radii mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. atomic and ionic radius. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. This page explains the various measures of atomic radius, and then looks at the way it varies. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. Radii of atoms and ions. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Typical values range from 30åpm (0.3åä) to over 200åpm. Although neither atoms nor ions have. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm.
From sciencetrends.com
Ionic Radius Trend Science Trends Magnesium Ions Radii Radii of atoms and ions. Although neither atoms nor ions have. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron. Magnesium Ions Radii.
From www.dreamstime.com
Diagram Representation of the Element Magnesium Stock Vector Magnesium Ions Radii Typical values range from 30åpm (0.3åä) to over 200åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. Radii of atoms and ions. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with. Magnesium Ions Radii.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Ions Radii This page explains the various measures of atomic radius, and then looks at the way it varies. Radii of atoms and ions. Although neither atoms nor ions have. Typical values range from 30åpm (0.3åä) to over 200åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that. Magnesium Ions Radii.
From chemistry.stackexchange.com
chemistry Why are hydrated lithium ions' radii larger than Magnesium Ions Radii Although neither atoms nor ions have. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. ionic radii are typically given in units of either picometers (pm) or angstroms (ä),. Magnesium Ions Radii.
From inspiritvr.com
Ionic radii Study Guide Inspirit Learning Inc Magnesium Ions Radii This page explains the various measures of atomic radius, and then looks at the way it varies. Although neither atoms nor ions have. atomic and ionic radius. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a. Magnesium Ions Radii.
From www.researchgate.net
Amounts of magnesium ions released from the magnesium ion modified Magnesium Ions Radii a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. atomic and ionic radius. This page explains the various measures of atomic radius, and then looks at the way it varies. Although neither atoms nor ions have. 100 rows ionic radius, rion, is the radius of a. Magnesium Ions Radii.
From www.slideserve.com
PPT Chemical Periodicity PowerPoint Presentation, free download ID Magnesium Ions Radii Typical values range from 30åpm (0.3åä) to over 200åpm. Although neither atoms nor ions have. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. atomic and ionic radius. mg is smaller. Magnesium Ions Radii.
From chemistry.stackexchange.com
atomic radius Why does Mg2+ have the ionic radii bigger than Ca2 Magnesium Ions Radii a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it. Magnesium Ions Radii.
From opentextbc.ca
2.4 Silicate Minerals Physical Geology Magnesium Ions Radii Radii of atoms and ions. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. atomic and ionic radius. 100. Magnesium Ions Radii.
From www.researchgate.net
The physical model schematic function in one specie of the magnesium Magnesium Ions Radii Radii of atoms and ions. atomic and ionic radius. This page explains the various measures of atomic radius, and then looks at the way it varies. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. ionic radii are typically given in units of either picometers (pm) or angstroms (ä),. Magnesium Ions Radii.
From www.chegg.com
Solved Refer to the table given for the ionic radii in your Magnesium Ions Radii 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. This page explains the various measures of atomic radius, and then looks at the way it varies. atomic and ionic radius. Although neither atoms nor ions have. ionic radii are typically given in units of either picometers (pm) or angstroms. Magnesium Ions Radii.
From mungfali.com
Magnesium Orbital Diagram Magnesium Ions Radii ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. atomic and ionic radius. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. This page explains the various measures of atomic radius, and then looks at the way it varies. Typical values. Magnesium Ions Radii.
From www.researchgate.net
Amounts of magnesium ions released from the magnesium ion modified Magnesium Ions Radii Typical values range from 30åpm (0.3åä) to over 200åpm. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. This page explains the various measures of atomic radius, and then looks at the way it varies. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with. Magnesium Ions Radii.
From www.flexiprep.com
NCERT Class 9 Science Solutions Chapter 3 Atoms and Molecules Part 9 Magnesium Ions Radii This page explains the various measures of atomic radius, and then looks at the way it varies. Typical values range from 30åpm (0.3åä) to over 200åpm. Radii of atoms and ions. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. 100 rows ionic radius, rion, is the. Magnesium Ions Radii.
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector Magnesium Ions Radii ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. atomic and ionic radius. Typical values range from 30åpm (0.3åä) to over 200åpm. Although neither atoms nor ions have. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. mg is smaller. Magnesium Ions Radii.
From www.semanticscholar.org
Figure 1 from Effect of magnesium ions on the inhibition of S Magnesium Ions Radii ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. mg is. Magnesium Ions Radii.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Magnesium Ions Radii atomic and ionic radius. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. Typical values range from 30åpm (0.3åä) to over 200åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron. Magnesium Ions Radii.
From employees.csbsju.edu
Structure & Reactivity Appendix Periodic Ionic Radii Magnesium Ions Radii This page explains the various measures of atomic radius, and then looks at the way it varies. Typical values range from 30åpm (0.3åä) to over 200åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. 100 rows ionic. Magnesium Ions Radii.
From chem.libretexts.org
7.3 Sizes of Atoms and Ions Chemistry LibreTexts Magnesium Ions Radii 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. atomic and ionic radius. mg is smaller the ca because it is above it in the periodic table, magnesium. Magnesium Ions Radii.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Ions Radii atomic and ionic radius. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. ionic radii are typically given in units of either. Magnesium Ions Radii.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ions Radii Typical values range from 30åpm (0.3åä) to over 200åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms. Magnesium Ions Radii.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Ions Radii Typical values range from 30åpm (0.3åä) to over 200åpm. Radii of atoms and ions. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. mg is smaller. Magnesium Ions Radii.
From www.crystalmaker.com
Elements, Atomic Radii and the Periodic Radii Magnesium Ions Radii This page explains the various measures of atomic radius, and then looks at the way it varies. Typical values range from 30åpm (0.3åä) to over 200åpm. atomic and ionic radius. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have. mg is smaller the. Magnesium Ions Radii.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium Ions Radii 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Radii of atoms and ions. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. ionic radii are typically given in. Magnesium Ions Radii.
From www.youtube.com
Ionic Radius Trends, Basic Introduction, Periodic Table, Sizes of Magnesium Ions Radii mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. Radii of atoms and ions. atomic and ionic radius. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. Typical values. Magnesium Ions Radii.
From chem.libretexts.org
Chapter 4.2 Lattice Energies in Ionic Solids Chemistry LibreTexts Magnesium Ions Radii a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. atomic and ionic radius. Radii of atoms and ions. 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. ionic radii are typically given in units of either picometers. Magnesium Ions Radii.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Magnesium Ions Radii Radii of atoms and ions. atomic and ionic radius. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is. Magnesium Ions Radii.
From www.animalia-life.club
Ionic Radius Diagram Magnesium Ions Radii 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Typical values range from 30åpm (0.3åä) to over 200åpm. Although neither atoms nor ions have. atomic and ionic radius. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. Radii. Magnesium Ions Radii.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Magnesium Ions Radii a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. This page explains the various measures of atomic radius, and then looks at the way it varies. Radii of atoms and. Magnesium Ions Radii.
From www.mdpi.com
IJMS Free FullText Theoretical Calculation of Absolute Radii of Magnesium Ions Radii This page explains the various measures of atomic radius, and then looks at the way it varies. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has. Magnesium Ions Radii.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Ions Radii Although neither atoms nor ions have. Typical values range from 30åpm (0.3åä) to over 200åpm. ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. Radii of atoms and ions. atomic and ionic radius. mg is smaller the ca because it is above it in the periodic table, magnesium being. Magnesium Ions Radii.
From www.scienceforums.net
Chemistry Comparing Ionic Radii of Cs+ and Cl ions Homework Help Magnesium Ions Radii 100 rows ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Typical values range from 30åpm (0.3åä) to over 200åpm. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. atomic and ionic radius. Radii of atoms and ions. Although neither. Magnesium Ions Radii.
From mungfali.com
Largest Atomic Radius Periodic Table Periodic Table Timeline CDF Magnesium Ions Radii atomic and ionic radius. Typical values range from 30åpm (0.3åä) to over 200åpm. This page explains the various measures of atomic radius, and then looks at the way it varies. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell. Magnesium Ions Radii.
From neetlab.com
Ionic Radius NEET Lab Magnesium Ions Radii ionic radii are typically given in units of either picometers (pm) or angstroms (ä), with 1åäå= 100åpm. mg is smaller the ca because it is above it in the periodic table, magnesium being a period above it shows that it has one less electron shell so. atomic and ionic radius. Radii of atoms and ions. 100. Magnesium Ions Radii.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Magnesium Oxide MgO Stock Illustration Magnesium Ions Radii atomic and ionic radius. Typical values range from 30åpm (0.3åä) to over 200åpm. Although neither atoms nor ions have. This page explains the various measures of atomic radius, and then looks at the way it varies. a comparison of ionic radii with atomic radii (figure \(\pageindex{7}\)) shows that a cation, having lost an electron, is always. 100. Magnesium Ions Radii.