An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide . (i) the electronic configuration of element x is : If the atomic number of an element x is 13; Write the formula of the. Hence, the formula of its oxide will be x 2o. Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the modern periodic table and how valency of an element is calculated on the basis of its atomic number. An element x combines with oxygen to form an oxide xo. Atomic number of the element = 16 thus, electronic configuration. Complete step by step answer: The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. Element x has atomic number 19. This oxide is electrically con¬ducting. Its valency will be one. (ii) the formula of the oxide 'x' is x 2 o. The number of electrons in its ion x 3 + will be 10. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps:
from www.adda247.com
Hence, the formula of its oxide will be x 2o. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: This is because x 3 + is a positively charged cation. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. This oxide is electrically con¬ducting. Element x has atomic number 19. Write the formula of the. Atomic number of the element = 16 thus, electronic configuration. Complete step by step answer: The number of electrons in its ion x 3 + will be 10.
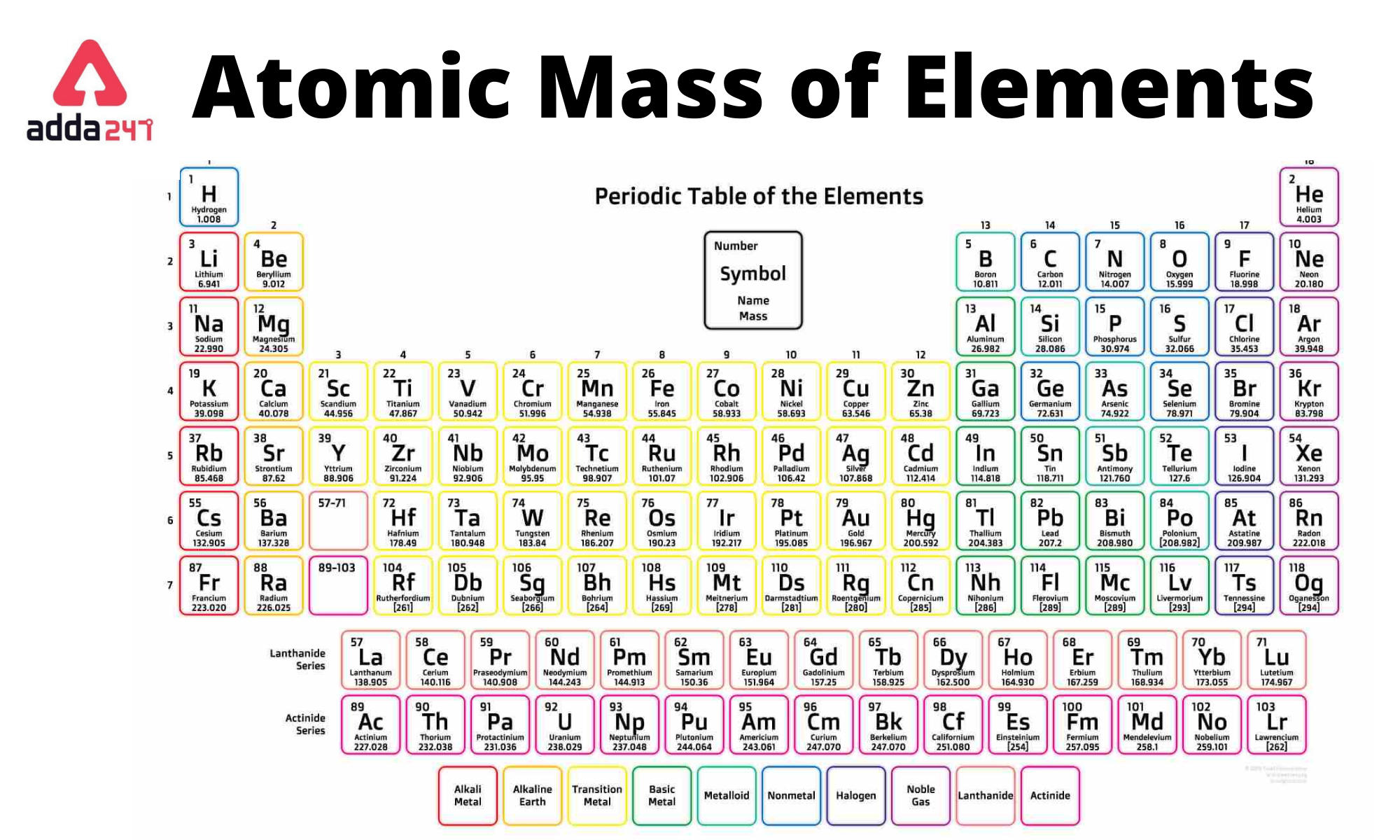
Atomic Mass of Elements 1 to 30 with Symbols PDF Download
An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Complete step by step answer: If the atomic number of an element x is 13; The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. This oxide is electrically con¬ducting. Element x has atomic number 19. (i) the electronic configuration of element x is : (ii) the formula of the oxide 'x' is x 2 o. Hence, the formula of its oxide will be x 2o. Its valency will be one. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. The number of electrons in its ion x 3 + will be 10. This is because x 3 + is a positively charged cation. Write the formula of the. An element x combines with oxygen to form an oxide xo. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: Atomic number of the element = 16 thus, electronic configuration.
From brainly.in
List of 30 elements with atomic number and mass and valency Brainly.in An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide This is because x 3 + is a positively charged cation. Its valency will be one. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. The number of electrons in its ion x 3 + will be 10. Write the formula of the. Taking the example of an. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From brainly.in
an element X having Atomic Number 13 reacts with y HavingAtomic Number An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: The number of electrons in its ion x 3 + will be 10. (i) the electronic configuration of element x is : Its valency will be one. Taking the example of an element of atomic number 16, explain how the. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.chemicool.com
Periodic Table of Elements with Relative Atomic Masses An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide The number of electrons in its ion x 3 + will be 10. If the atomic number of an element x is 13; Element x has atomic number 19. Atomic number of the element = 16 thus, electronic configuration. (ii) the formula of the oxide 'x' is x 2 o. The atom calculator is a tool for calculating the atomic. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide The number of electrons in its ion x 3 + will be 10. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: If the atomic number of an element x is 13; (i) the electronic configuration of element x is : Its valency will be one. Atomic number of. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From chem.libretexts.org
4.5 Chemical Symbols and the Atomic Number Chemistry LibreTexts An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide This is because x 3 + is a positively charged cation. Hence, the formula of its oxide will be x 2o. Write the formula of the. This oxide is electrically con¬ducting. The number of electrons in its ion x 3 + will be 10. Atomic number of the element = 16 thus, electronic configuration. If the atomic number of an. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.chegg.com
Solved Mystery element X has the electron energy levels An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. (ii) the formula of the oxide 'x' is x 2 o. Complete step by step answer: Write the formula of the. This oxide is electrically con¬ducting. Element x has atomic number 19. If the atomic number of. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From reviewhomedecor.co
First 20 Elements Of The Periodic Table With Atomic Number And Mass An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide (ii) the formula of the oxide 'x' is x 2 o. An element x combines with oxygen to form an oxide xo. Hence, the formula of its oxide will be x 2o. The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. (i) the electronic configuration of. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From mungfali.com
Periodic Table Of Elements With Atomic Weight An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Its valency will be one. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the modern periodic table and how valency of. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From periodictable.me
Printable Periodic Table With Atomic Number An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide (ii) the formula of the oxide 'x' is x 2 o. The number of electrons in its ion x 3 + will be 10. This oxide is electrically con¬ducting. Complete step by step answer: Write the formula of the. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: (i). An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.meritnation.com
Three elements 'X', 'Y' and 'Z' have atomic numbers 7, 8 and 9 An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide (ii) the formula of the oxide 'x' is x 2 o. Complete step by step answer: The number of electrons in its ion x 3 + will be 10. Element x has atomic number 19. Atomic number of the element = 16 thus, electronic configuration. Its valency will be one. An element x combines with oxygen to form an oxide. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.thoughtco.com
Element List Atomic Number, Element Name and Symbol An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: (i) the electronic configuration of element x is : Hence, the formula of its oxide will be x 2o. If the atomic number of an element x is 13; Taking the example of an element of atomic number 16, explain. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From itc.gsw.edu
Atomic Structure An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide (ii) the formula of the oxide 'x' is x 2 o. (i) the electronic configuration of element x is : Atomic number of the element = 16 thus, electronic configuration. Element x has atomic number 19. If the atomic number of an element x is 13; The number of electrons in its ion x 3 + will be 10. Complete. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From stock.adobe.com
Sulfur atomic structure has atomic number, atomic mass, electron An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide An element x combines with oxygen to form an oxide xo. This is because x 3 + is a positively charged cation. The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. Its valency will be one. The number of electrons in its ion x 3 +. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.pinterest.com
RS_AtomicSymbol.gif (1024×768) Mass number, Atomic number, Science notes An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide This oxide is electrically con¬ducting. Element x has atomic number 19. This is because x 3 + is a positively charged cation. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: The element x has atomic number = 16 and as we know the atomic number is equal to. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From iperiodictable.com
Printable Periodic Table With Names, Atomic Mass or Number An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Atomic number of the element = 16 thus, electronic configuration. Write the formula of the. (i) the electronic configuration of element x is : If the atomic number of an element x is 13; To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: This is because x 3 +. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide If the atomic number of an element x is 13; The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. This is because x 3 + is a positively charged cation. Write the formula of the. Taking the example of an element of atomic number 16, explain how the. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From mungfali.com
Periodic Table Rounded Atomic Mass An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the modern periodic table and how valency of an element is calculated on the basis of its atomic number. Element x has atomic number 19. (ii) the formula of the oxide 'x' is x. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide An element x combines with oxygen to form an oxide xo. Write the formula of the. (i) the electronic configuration of element x is : Element x has atomic number 19. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: Complete step by step answer: Taking the example of. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide This oxide is electrically con¬ducting. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. Atomic number of the element = 16 thus, electronic configuration. (i). An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From biosynthesisthomas.blogspot.com
BioSynthesis Chapter 2 The Chemical Basis of Life I Atoms, Molecules An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide (i) the electronic configuration of element x is : Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the modern periodic table and how valency of an element is calculated on the basis of its atomic number. Its valency will be one. Atomic. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide If the atomic number of an element x is 13; Element x has atomic number 19. (ii) the formula of the oxide 'x' is x 2 o. The number of electrons in its ion x 3 + will be 10. Hence, the formula of its oxide will be x 2o. This oxide is electrically con¬ducting. Atomic number of the element. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Atomic number of the element = 16 thus, electronic configuration. To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: Element x has atomic number 19. Write the formula of the. Complete step by step answer: This is because x 3 + is a positively charged cation. If the atomic. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From brokeasshome.com
Periodic Table Of Elements With Names And Symbols Atomic Mass Number In An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide The number of electrons in its ion x 3 + will be 10. If the atomic number of an element x is 13; To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: The element x has atomic number = 16 and as we know the atomic number is equal. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definition [with Examples] An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide This oxide is electrically con¬ducting. Write the formula of the. Hence, the formula of its oxide will be x 2o. (i) the electronic configuration of element x is : Element x has atomic number 19. Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From periodictable.me
Atomic Number Examples Archives Dynamic Periodic Table of Elements An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Element x has atomic number 19. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. The number of electrons in its ion x 3 + will be 10. Its valency will be one. (i) the electronic configuration of element x is : To determine the formula of the. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide If the atomic number of an element x is 13; This oxide is electrically con¬ducting. The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. Atomic number of the element = 16 thus, electronic configuration. The number of electrons in its ion x 3 + will be. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.javatpoint.com
Definition of Atomic Number JavaTpoint An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide An element x combines with oxygen to form an oxide xo. Write the formula of the. Hence, the formula of its oxide will be x 2o. The number of electrons in its ion x 3 + will be 10. Complete step by step answer: This oxide is electrically con¬ducting. This is because x 3 + is a positively charged cation.. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From brainly.in
an element X has atomic mass 27 and atomic number 13 first draw the An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide This oxide is electrically con¬ducting. If the atomic number of an element x is 13; The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. This. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From sciencenotes.org
List of Elements By Atomic Number An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Hence, the formula of its oxide will be x 2o. This oxide is electrically con¬ducting. (ii) the formula of the oxide 'x' is x 2 o. The number of electrons in its ion x 3 + will be 10. Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.quia.com
Quia Atoms An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Hence, the formula of its oxide will be x 2o. If the atomic number of an element x is 13; (i) the electronic configuration of element x is : The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. The atom calculator is a tool for calculating. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From www.adda247.com
Atomic Mass of Elements 1 to 30 with Symbols PDF Download An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide To determine the formula of the oxide of the element x with atomic number 19, we can follow these steps: Its valency will be one. (i) the electronic configuration of element x is : The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. Complete step by. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From courses.lumenlearning.com
Properties of Elements Biology for NonMajors I An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide Its valency will be one. The number of electrons in its ion x 3 + will be 10. Atomic number of the element = 16 thus, electronic configuration. Write the formula of the. (i) the electronic configuration of element x is : The element x has atomic number = 16 and as we know the atomic number is equal to. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From schematiclistmorvant.z13.web.core.windows.net
Labelled Diagram Of Helium Atom An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide This oxide is electrically con¬ducting. Complete step by step answer: This is because x 3 + is a positively charged cation. The number of electrons in its ion x 3 + will be 10. The element x has atomic number = 16 and as we know the atomic number is equal to the number of protons which. If the atomic. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From sustainablegros.weebly.com
Periodic table atomic number superscript sustainableGros An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide (i) the electronic configuration of element x is : This is because x 3 + is a positively charged cation. The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. The number of electrons in its ion x 3 + will be 10. Element x has atomic number 19.. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.
From sgwebdigital.com
Chemische elementen Atomen SG An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom. The number of electrons in its ion x 3 + will be 10. Complete step by step answer: Element x has atomic number 19. Atomic number of the element = 16 thus, electronic configuration. This is because x 3. An Element X Has Atomic Number 16 What Will Be The Formula Of Its Oxide.