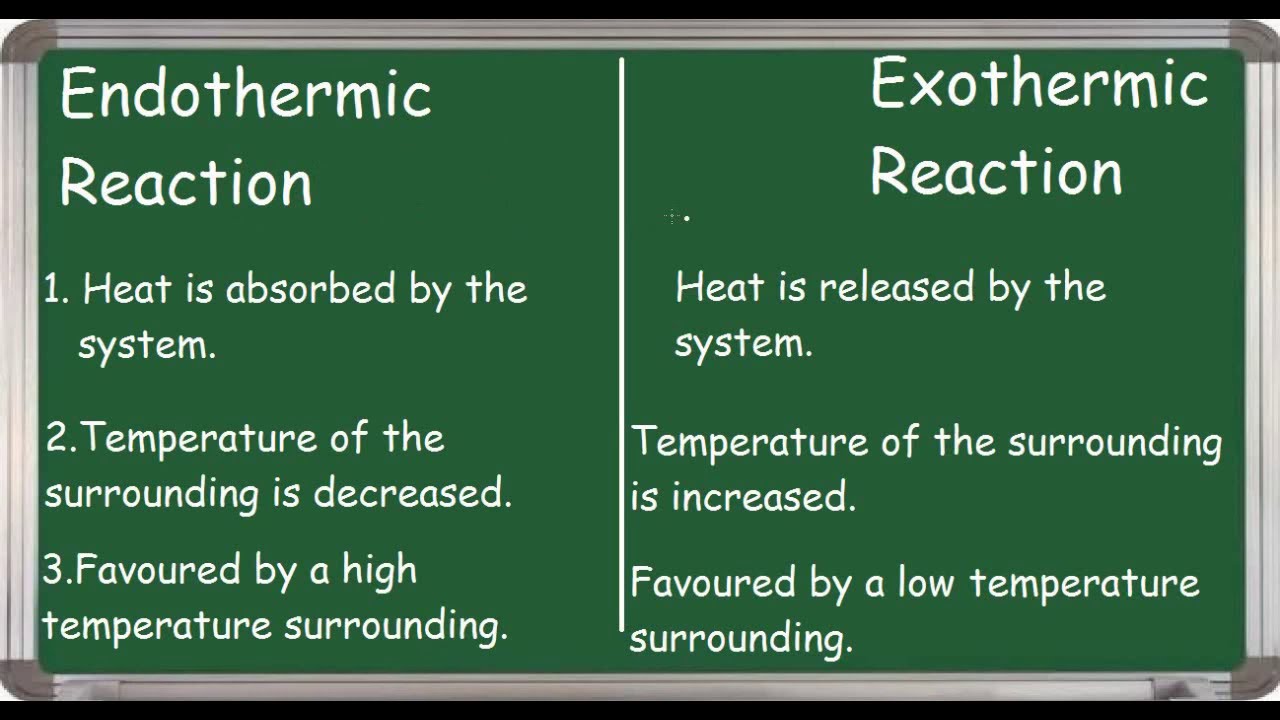
Is Endo Positive Or Negative . how can energy change. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. atoms are held together by a certain amount of energy called bond energy. Exothermic, endothermic, & chemical change. Energy is required to break bonds. Energy is released when chemical bonds are. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. in the profile for an endothermic reaction, the overall change is positive. You can tell this because the products have more energy. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)).
from learningschoolsky17276p.z4.web.core.windows.net
how can energy change. Exothermic, endothermic, & chemical change. Energy is released when chemical bonds are. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. Energy is required to break bonds. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. atoms are held together by a certain amount of energy called bond energy.
Endothermic And Exothermic Reaction Worksheet
Is Endo Positive Or Negative how can energy change. atoms are held together by a certain amount of energy called bond energy. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. Energy is released when chemical bonds are. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. You can tell this because the products have more energy. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. Energy is required to break bonds. Exothermic, endothermic, & chemical change. in the profile for an endothermic reaction, the overall change is positive. how can energy change.
From exoupmxhr.blob.core.windows.net
Endodontics Exam Quizlet at Kathy Wilson blog Is Endo Positive Or Negative as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. atoms are held together by a certain amount of energy called bond energy. Exothermic, endothermic, & chemical change. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. Energy is required to break bonds. You can tell this because the products have more energy. to summarise, for. Is Endo Positive Or Negative.
From printabledokkenbk.z21.web.core.windows.net
Endothermic And Exothermic Explained Is Endo Positive Or Negative to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. atoms are held together by a certain amount of energy called bond energy. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). You can tell this because the products have more energy. . Is Endo Positive Or Negative.
From thechemistrynotes.com
Differences Between Exothermic and Endothermic Reactions Is Endo Positive Or Negative You can tell this because the products have more energy. how can energy change. in the profile for an endothermic reaction, the overall change is positive. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. endothermic reactions are characterized by positive heat flow (into the. Is Endo Positive Or Negative.
From www.oralhealthgroup.com
Misadventures in Endodontics Turning a Negative into a Positive Oral Is Endo Positive Or Negative Exothermic, endothermic, & chemical change. Energy is required to break bonds. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). in the profile for an endothermic reaction, the overall change is positive. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. . Is Endo Positive Or Negative.
From learningdarkvonhack2n.z21.web.core.windows.net
Real Life Example Of An Exothermic Reaction Is Endo Positive Or Negative endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. You can tell this because the products have more energy. Energy is required to break bonds. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. atoms are held together by. Is Endo Positive Or Negative.
From lessonlibraryfettes.z22.web.core.windows.net
Endothermic Reactions And Exothermic Reaction Is Endo Positive Or Negative Energy is required to break bonds. Energy is released when chemical bonds are. how can energy change. in the profile for an endothermic reaction, the overall change is positive. You can tell this because the products have more energy. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. to. Is Endo Positive Or Negative.
From www.youtube.com
Advance Your Endo with Apical Negative Pressure Irrigation YouTube Is Endo Positive Or Negative atoms are held together by a certain amount of energy called bond energy. Energy is required to break bonds. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. You can tell this because the products have more energy. how can energy change. to summarise, for. Is Endo Positive Or Negative.
From www.osakadental.com
Dental Negative Pressure Endo Irrigation System Buy Dental Irrigation Is Endo Positive Or Negative to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. atoms are held together by a certain amount of energy called bond energy. Energy is required to break bonds. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Conversely, a positive [latex]\ce{\delta h}[/latex]. Is Endo Positive Or Negative.
From www.youtube.com
VET ENDO Shortloop and Longloop Negative Feedback Student Output Is Endo Positive Or Negative Energy is required to break bonds. Exothermic, endothermic, & chemical change. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. how can energy change. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. You can tell this because the products have more. Is Endo Positive Or Negative.
From cekapazk.blob.core.windows.net
What Is Endo In Dentistry at Courtney Warman blog Is Endo Positive Or Negative You can tell this because the products have more energy. atoms are held together by a certain amount of energy called bond energy. Exothermic, endothermic, & chemical change. in the profile for an endothermic reaction, the overall change is positive. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can. Is Endo Positive Or Negative.
From mungfali.com
Gram Positive Cell Wall Diagram Is Endo Positive Or Negative Energy is released when chemical bonds are. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. Exothermic, endothermic, & chemical change. in the profile for an endothermic reaction, the overall change is positive. as such, a. Is Endo Positive Or Negative.
From www.accessdentallearning.com
Endodontics — Access Is Endo Positive Or Negative Energy is required to break bonds. in the profile for an endothermic reaction, the overall change is positive. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). to summarise, for an endothermic change, {eq}\delta h {/eq}. Is Endo Positive Or Negative.
From www.researchgate.net
(PDF) Individual differences in response to positive and negative Is Endo Positive Or Negative You can tell this because the products have more energy. how can energy change. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. Exothermic, endothermic, & chemical change. atoms are held together by a certain amount of energy called bond energy. endothermic reactions are characterized. Is Endo Positive Or Negative.
From myriadgenetics.eu
Node negative breast cancer recurrence Myriad International Is Endo Positive Or Negative Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. how can energy change. in the profile for an endothermic reaction, the overall change is positive. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. Energy is released when chemical bonds are. endothermic reactions are characterized by positive. Is Endo Positive Or Negative.
From www.aiclinic.or.kr
SmartEndoDetail Is Endo Positive Or Negative Energy is required to break bonds. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. in the profile for an endothermic reaction, the overall change is positive. You can tell this because the products have more energy. atoms are held together by a certain amount of energy called bond energy.. Is Endo Positive Or Negative.
From www.slideserve.com
PPT By Jennifer Joiner PowerPoint Presentation, free download ID Is Endo Positive Or Negative endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. Exothermic,. Is Endo Positive Or Negative.
From describingword.com
Top 30 Adjectives for Endo (Negative & Positive Words) Is Endo Positive Or Negative as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. in the profile for an endothermic reaction, the overall change is positive. Energy is released when chemical bonds are. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. how. Is Endo Positive Or Negative.
From www.mdpi.com
Applied Sciences Free FullText Advancing Photodynamic Therapy for Is Endo Positive Or Negative Energy is required to break bonds. in the profile for an endothermic reaction, the overall change is positive. Exothermic, endothermic, & chemical change. atoms are held together by a certain amount of energy called bond energy. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. . Is Endo Positive Or Negative.
From apsjournals.apsnet.org
CrpLike Protein Plays Both Positive and Negative Roles in Regulating Is Endo Positive Or Negative how can energy change. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. atoms are held together by a certain amount of energy called bond energy. Exothermic, endothermic, & chemical change. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. You can tell this because the products have. Is Endo Positive Or Negative.
From www.livingwithendometriosis.org
Living With Endometriosis » Post Topic » Endo Awareness Month Does Is Endo Positive Or Negative Energy is released when chemical bonds are. atoms are held together by a certain amount of energy called bond energy. how can energy change. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. as such, a negative [latex]\ce{\delta h}[/latex] informs us that. Is Endo Positive Or Negative.
From learningschoolsky17276p.z4.web.core.windows.net
Endothermic And Exothermic Reaction Worksheet Is Endo Positive Or Negative Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. You can tell this because the products have more energy. Energy is released when chemical bonds are. . Is Endo Positive Or Negative.
From printableabsolutefreakmn.z14.web.core.windows.net
Endothermic And Exothermic Explained Is Endo Positive Or Negative atoms are held together by a certain amount of energy called bond energy. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. You can tell this because the products have more energy. Energy is required to break bonds. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in. Is Endo Positive Or Negative.
From www.youtube.com
Causes of Endodontic Failure YouTube Is Endo Positive Or Negative how can energy change. Energy is released when chemical bonds are. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. Energy is required to break bonds. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. You can tell this because the products have more energy. in the profile. Is Endo Positive Or Negative.
From www.youtube.com
Ledge in Endodontics Root Canal What, Causes, Prevention, Removal Is Endo Positive Or Negative Energy is released when chemical bonds are. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. atoms are held together by a certain amount of energy called bond energy. You. Is Endo Positive Or Negative.
From studylib.net
endo vs implant Text only Clinical Jude Is Endo Positive Or Negative Energy is released when chemical bonds are. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). how can energy change. atoms are held together by a certain amount of energy called bond. Is Endo Positive Or Negative.
From www.vrogue.co
What Is Enthalpy Chemistry Steps vrogue.co Is Endo Positive Or Negative You can tell this because the products have more energy. atoms are held together by a certain amount of energy called bond energy. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Energy is released when chemical bonds are. . Is Endo Positive Or Negative.
From www.researchgate.net
Summary of the advantages and disadvantages of the regenerative Is Endo Positive Or Negative Energy is released when chemical bonds are. Energy is required to break bonds. Exothermic, endothermic, & chemical change. atoms are held together by a certain amount of energy called bond energy. You can tell this because the products have more energy. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. how can energy. Is Endo Positive Or Negative.
From europepmc.org
Static vs. dynamic navigation for endodontic microsurgery A Is Endo Positive Or Negative endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. atoms are held together by a certain amount of energy called bond energy. Energy is required to break bonds. Exothermic, endothermic, & chemical change.. Is Endo Positive Or Negative.
From www.mdpi.com
Bioengineering Free FullText Towards a New Concept of Regenerative Is Endo Positive Or Negative You can tell this because the products have more energy. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Energy is released when chemical bonds are. Exothermic, endothermic, & chemical change. Energy is required to break bonds. endothermic reactions are. Is Endo Positive Or Negative.
From www.pall.com
Endotoxin Removal Medical Pall Corporation Is Endo Positive Or Negative atoms are held together by a certain amount of energy called bond energy. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. Energy is released when chemical bonds are. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). You can tell this because the products have. Is Endo Positive Or Negative.
From www.dentalfry.com
Single Visit Endodontics Advantages, Disadvantages, Indications Is Endo Positive Or Negative in the profile for an endothermic reaction, the overall change is positive. Energy is required to break bonds. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. to summarise, for an endothermic change, {eq}\delta h {/eq} is always positive but {eq}\delta s {/eq} can be, positive in. how can energy change. . Is Endo Positive Or Negative.
From blog.naver.com
[Sepsis (2)] Sepsis를 애매하게 알고 있다면, 병태생리 네이버 블로그 Is Endo Positive Or Negative Energy is released when chemical bonds are. Energy is required to break bonds. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. how can energy change. You can tell this because the products have more energy. in the profile for an endothermic reaction, the overall change is positive. as. Is Endo Positive Or Negative.
From www.microbiologyinpictures.com
Endo agar plate with Pseudomonas aeruginosa and Escherichia coli Is Endo Positive Or Negative endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. Energy is required to break bonds. in the profile for an endothermic reaction, the overall change is positive. Conversely, a positive [latex]\ce{\delta h}[/latex] indicates an. You can tell this because the products have more energy. to summarise, for an endothermic change,. Is Endo Positive Or Negative.
From decisionsindentistry.com
Universal Classification in Endodontic Diagnosis Decisions in Dentistry Is Endo Positive Or Negative You can tell this because the products have more energy. as such, a negative [latex]\ce{\delta h}[/latex] informs us that the reaction is exothermic. atoms are held together by a certain amount of energy called bond energy. how can energy change. Exothermic, endothermic, & chemical change. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn}. Is Endo Positive Or Negative.
From margaretghopkennedy.blogspot.com
Which of the Following Is Not an Endotoxin Is Endo Positive Or Negative how can energy change. endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Energy is required to break bonds. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy. You can tell this because the products have more energy. to summarise, for an endothermic change, {eq}\delta. Is Endo Positive Or Negative.