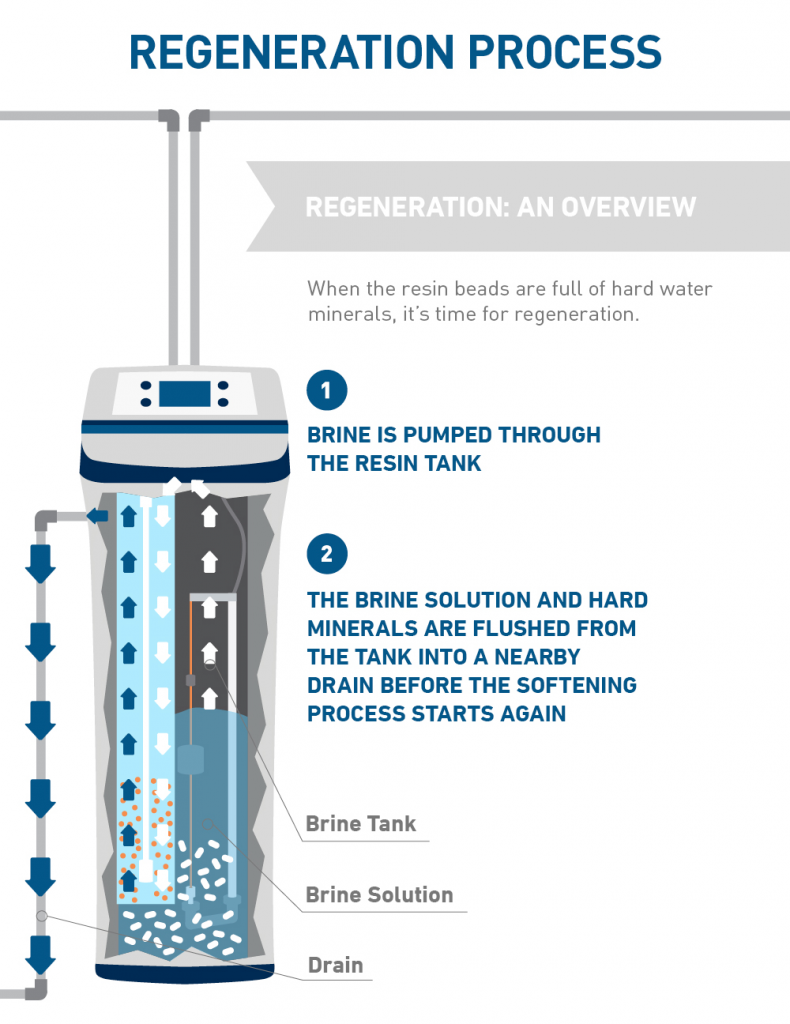
Water Softener And Chemicals . On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and.
from homewater101.com
A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Our framework provides a better understanding of softening and can prepare water utility planners and managers for better.
How Does a Water Softener Work? Water Softening Process Diagram
Water Softener And Chemicals On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. A water softener is a type of water treatment system that removes hardness minerals from a water supply.
From www.wpsexpert.com
3 Stage Water softener with Fleck 5600 SXT Control Valve Plumbing Water Softener And Chemicals Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate. Water Softener And Chemicals.
From www.corodexindustries.com
Water Softeners Corodex Industries Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Water softening is achieved. Water Softener And Chemicals.
From antunes.com
Water Softener Treatments Water Softener Diagram Antunes Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners. Water Softener And Chemicals.
From watertechadvice.com
How Does a Water Softener Work? (What it is & What it Does Explained) Water Softener And Chemicals Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Our framework provides a better understanding of softening and can prepare water utility planners. Water Softener And Chemicals.
From www.walmart.com
Aquasure Whole House Filtration with 48,000 Grains Water Softener Water Softener And Chemicals Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Our framework provides a better understanding of softening and can prepare water utility planners. Water Softener And Chemicals.
From pearlwater.in
Top 5 Water Softeners For Your Home Water Softener And Chemicals On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers. Water Softener And Chemicals.
From www.rowatertreatment-system.com
Drinking Water Softener System 2000LPH For Chemical Industry CE Approved Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. A water softener is a type of water treatment system that removes hardness minerals. Water Softener And Chemicals.
From netsolwater.com
Why use of water softener plant in chemical laboratories Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates. Water Softener And Chemicals.
From jeiaquatech.in
Water Softener With Sand Filter In Better Water Filtration Water Softener And Chemicals Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum. Water Softener And Chemicals.
From www.indiamart.com
Water Softener Chemical at Rs 88/kilogram Water Softener Chemical in Water Softener And Chemicals Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening can be accomplished through the addition. Water Softener And Chemicals.
From userlibfrei.z19.web.core.windows.net
Manual Regeneration Water Softener Water Softener And Chemicals Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. A water softener is a type of water treatment system that removes hardness minerals from a. Water Softener And Chemicals.
From homewater101.com
How Does a Water Softener Work? Water Softening Process Diagram Water Softener And Chemicals Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. A water softener is a type of water treatment system that removes hardness minerals from a water supply. On a small scale, chemicals used. Water Softener And Chemicals.
From www.austinwatersolutions.net
A Complete Guide to Water Softeners Austin Water Solutions Water Softener And Chemicals A water softener is a type of water treatment system that removes hardness minerals from a water supply. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. On a small scale, chemicals used for softening include ammonia , borax , calcium. Water Softener And Chemicals.
From allchemical.com.au
Softener filter with Automatic/Manual Valve All Chemical Water Softener And Chemicals Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Our framework provides a better understanding of softening. Water Softener And Chemicals.
From www.alkalinewaterpoint.com
Reverse Osmosis Vs Water Softener Advantages and Disadvantages Water Softener And Chemicals A water softener is a type of water treatment system that removes hardness minerals from a water supply. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates. Water Softener And Chemicals.
From tinyhousegarage.com
How Does Water Softener Work Chemistry? Water Softener And Chemicals On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. Water softening is achieved either by adding chemicals that form insoluble precipitates or by. Water Softener And Chemicals.
From generationwatersystems.com
GenSoft EliteDual Commercial Grade Water Softener Generation Water Water Softener And Chemicals A water softener is a type of water treatment system that removes hardness minerals from a water supply. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. Water softening is achieved either by. Water Softener And Chemicals.
From homewater101.com
How Does a Water Softener Work? Water Softening Process Diagram Water Softener And Chemicals On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. The purpose of water softening is to remove all hardness minerals from water, preventing. Water Softener And Chemicals.
From www.korgentech.com
Water Softeners Water Softening Plants Water Softener And Chemicals A water softener is a type of water treatment system that removes hardness minerals from a water supply. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. On a small scale, chemicals used for softening include ammonia , borax , calcium. Water Softener And Chemicals.
From www.thespruce.com
All About Water Softeners and How They Work Water Softener And Chemicals On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening is achieved either by adding chemicals that form insoluble precipitates or by. Water Softener And Chemicals.
From www.indiamart.com
Industrial Water Softening Chemical at Rs 180 Water Softener Chemical Water Softener And Chemicals A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. The purpose of water softening is to remove. Water Softener And Chemicals.
From www.korgentech.com
Water Softeners Water Softening Plants Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. A water softener is a type of water treatment system that removes hardness minerals. Water Softener And Chemicals.
From www.clearwatersystems.com
SmartSoft Water Softeners Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. Water softening is achieved either by adding chemicals that form insoluble precipitates or by. Water Softener And Chemicals.
From www.purisoftwater.com
Chlor A Soft Water Softener with Chlorine Removal Purisoft Water Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked. Water Softener And Chemicals.
From www.whirlpoolwatersolutions.com
Hybrid Home Water Softener & Filtration System Whirlpool Water Softener And Chemicals Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening can be accomplished through the addition. Water Softener And Chemicals.
From aquaclearws.com
Water Softener Systems Aqua Clear Water Systems Water Softener And Chemicals The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Water softening can be. Water Softener And Chemicals.
From www.ecomfort.com
Water Softener Buyers Guide How to Pick the Best Water Softener Water Softener And Chemicals On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening is achieved either by adding chemicals that form insoluble precipitates or by. Water Softener And Chemicals.
From www.thespruce.com
6 Types of Water Softeners and How to Choose One Water Softener And Chemicals A water softener is a type of water treatment system that removes hardness minerals from a water supply. The purpose of water softening is to remove all hardness minerals from water, preventing the effects of hard water (such as scale deposits, sticky soap scum skin, and. On a small scale, chemicals used for softening include ammonia , borax , calcium. Water Softener And Chemicals.
From complete-water.com
Industrial Water Softener Parts, Benefits, CWS Water Softener And Chemicals Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. A water softener is a type of water treatment system that removes hardness minerals from a water supply. The purpose of water softening is. Water Softener And Chemicals.
From waterseer.org
Water Conditioner Vs Water Softener What's The Difference? Water Softener And Chemicals Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). The purpose of water softening is to remove all hardness minerals from water, preventing. Water Softener And Chemicals.
From waterfilterguru.com
How to Remove Salt from Softened Water (Top 3 Methods) Water Softener And Chemicals A water softener is a type of water treatment system that removes hardness minerals from a water supply. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. On a small scale, chemicals used. Water Softener And Chemicals.
From www.indiamart.com
Water Softener Chemical at Rs 130 Water Softener Chemical in Gurgaon Water Softener And Chemicals On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a type of water treatment system that removes hardness minerals from a. Water Softener And Chemicals.
From wetheadmedia.com
RainSoft water softener Water Softener And Chemicals Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a type of water treatment system that removes hardness minerals from a water supply. The purpose of water softening is to remove. Water Softener And Chemicals.
From www.reverseosmosis.com
Chemicals for Water Treatment Water Softener Cleaners Water Softener And Chemicals Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. On a small scale, chemicals used for softening include ammonia , borax , calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Water softening can be accomplished through the addition of chemicals that form insoluble precipitates. Water Softener And Chemicals.
From www.lowes.com
AQUASURE Signature Elite Whole House Water Treatment System with 64,000 Water Softener And Chemicals Water softening can be accomplished through the addition of chemicals that form insoluble precipitates or through ion exchange. Our framework provides a better understanding of softening and can prepare water utility planners and managers for better. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a type of water. Water Softener And Chemicals.