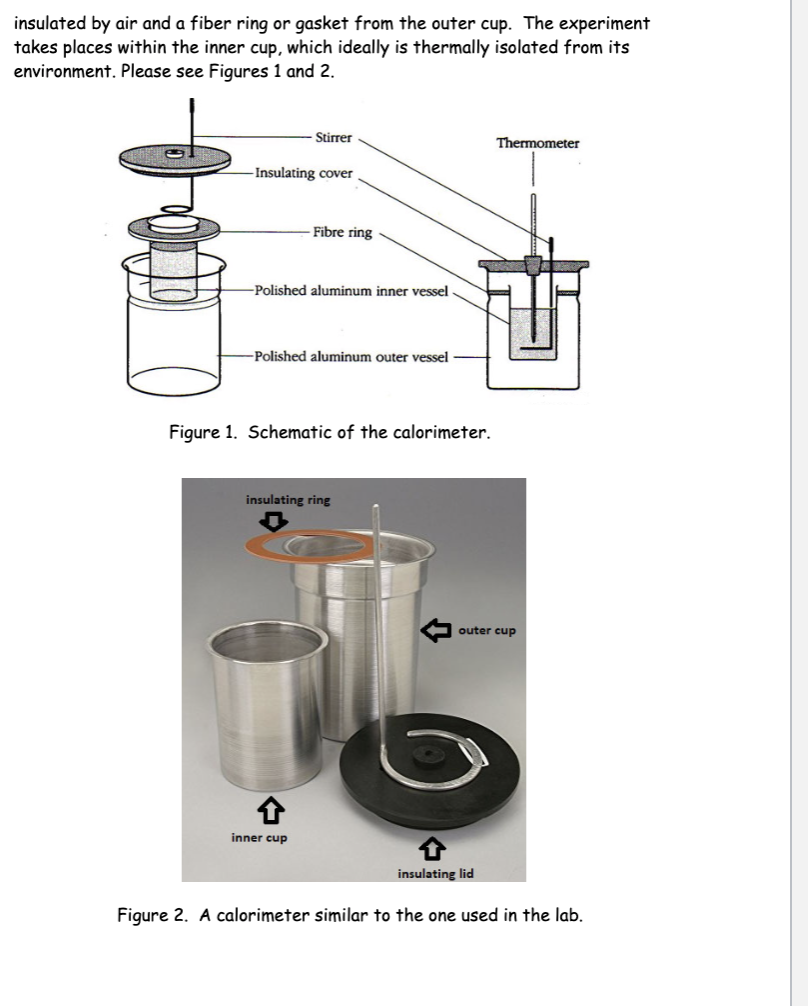
Calorimetry Can Be Used To Find Mark The Three That Are Correct . Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. We do this using simple calorimetry. Ex phys ch 5 study questions. Adding the enthalpy of reaction i and the enthalpy of reaction ii. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Which steps can be used to calculate the enthalpy of reaction iii correctly? One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Compare heat flow from hot to cold objects in an ideal. We can experimentally determine the relative amounts of energy released by a fuel. For example, when an exothermic. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees.
from www.chegg.com
Which steps can be used to calculate the enthalpy of reaction iii correctly? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. Adding the enthalpy of reaction i and the enthalpy of reaction ii. Apply the first law of thermodynamics to calorimetry. We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic.
Calorimetry is a technique that can be used to
Calorimetry Can Be Used To Find Mark The Three That Are Correct We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Ex phys ch 5 study questions. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Which steps can be used to calculate the enthalpy of reaction iii correctly? Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. We do this using simple calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Adding the enthalpy of reaction i and the enthalpy of reaction ii. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1875569 Calorimetry Can Be Used To Find Mark The Three That Are Correct Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Ex phys ch 5 study questions. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. Adding the enthalpy of reaction i and the enthalpy of reaction. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Can Be Used To Find Mark The Three That Are Correct A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Apply the first law of thermodynamics to calorimetry. We do this using simple calorimetry. Which. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From studylib.net
Calorimetry Calorimetry Can Be Used To Find Mark The Three That Are Correct Compare heat flow from hot to cold objects in an ideal. Which steps can be used to calculate the enthalpy of reaction iii correctly? Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Review for Exams YouTube Calorimetry Can Be Used To Find Mark The Three That Are Correct We do this using simple calorimetry. For example, when an exothermic. Ex phys ch 5 study questions. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Which steps can be used to calculate the enthalpy of reaction iii correctly? Where q is the amount of heat (in joules), c is. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Can Be Used To Find Mark The Three That Are Correct Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. We can experimentally determine the relative amounts of energy released by a fuel. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. We do this using simple calorimetry. Which. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.numerade.com
SOLVED Use the References to access important values if needed for this question. A bomb Calorimetry Can Be Used To Find Mark The Three That Are Correct We can experimentally determine the relative amounts of energy released by a fuel. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. We do this using simple calorimetry. Apply the first. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Can Be Used To Find Mark The Three That Are Correct Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. We can experimentally determine the relative amounts of energy released by a fuel. We do this using simple calorimetry. Adding the enthalpy of reaction i and the enthalpy of reaction ii. Compare heat flow from hot to cold objects in an ideal. Where q is. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Can Be Used To Find Mark The Three That Are Correct Ex phys ch 5 study questions. We can experimentally determine the relative amounts of energy released by a fuel. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. For example, when an exothermic. Adding the enthalpy of reaction i and the enthalpy of reaction ii. One technique we can use to measure. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.chegg.com
Solved Calorimetry can also be used to determine nutritional Calorimetry Can Be Used To Find Mark The Three That Are Correct For example, when an exothermic. We can experimentally determine the relative amounts of energy released by a fuel. Compare heat flow from hot to cold objects in an ideal. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From dxofzsmrb.blob.core.windows.net
Equation Used For Calorimetry at Devin Johnson blog Calorimetry Can Be Used To Find Mark The Three That Are Correct For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. Ex phys ch 5 study questions. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Where q is the amount of heat (in joules), c is the heat. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog Calorimetry Can Be Used To Find Mark The Three That Are Correct Adding the enthalpy of reaction i and the enthalpy of reaction ii. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. Which. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.studocu.com
Calorimetry Lab Report (Part 1) For this experiment, the definition of calorimetry will be Calorimetry Can Be Used To Find Mark The Three That Are Correct We can experimentally determine the relative amounts of energy released by a fuel. Which steps can be used to calculate the enthalpy of reaction iii correctly? Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. For example, when an exothermic. Compare. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calculate the Heat Calorimetry Can Be Used To Find Mark The Three That Are Correct For example, when an exothermic. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. Apply the first law of thermodynamics to calorimetry. Ex phys ch 5. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.chegg.com
Solved Calorimetry is a method used to measure changes in Calorimetry Can Be Used To Find Mark The Three That Are Correct Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. We do this using simple calorimetry. Adding the enthalpy of reaction i and the enthalpy of reaction ii. We can experimentally determine the relative amounts of energy released by a fuel. For example, when an exothermic. Where q is the amount of heat. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Can Be Used To Find Mark The Three That Are Correct Ex phys ch 5 study questions. Compare heat flow from hot to cold objects in an ideal. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. For example, when an exothermic. We do this using simple calorimetry. Calorimetry is used to measure the amount of thermal energy transferred. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Can Be Used To Find Mark The Three That Are Correct Compare heat flow from hot to cold objects in an ideal. Which steps can be used to calculate the enthalpy of reaction iii correctly? Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. Apply the first law of thermodynamics to calorimetry.. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Can Be Used To Find Mark The Three That Are Correct Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Ex phys ch 5 study questions. Which steps can be used to calculate the enthalpy of reaction iii correctly? For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Adding the enthalpy of reaction i and the enthalpy of reaction ii. A. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Calorimetry Can Be Used To Find Mark The Three That Are Correct We do this using simple calorimetry. Adding the enthalpy of reaction i and the enthalpy of reaction ii. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. We can experimentally determine the relative amounts of energy released by a fuel. One. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Can Be Used To Find Mark The Three That Are Correct Apply the first law of thermodynamics to calorimetry. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Adding the enthalpy of reaction i and. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch Calorimetry Can Be Used To Find Mark The Three That Are Correct Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. We do this using simple calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Adding the. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.chegg.com
Solved A bomb calorimeter can be used to measure the Calorimetry Can Be Used To Find Mark The Three That Are Correct We can experimentally determine the relative amounts of energy released by a fuel. Ex phys ch 5 study questions. Compare heat flow from hot to cold objects in an ideal. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Adding the enthalpy of reaction i and the enthalpy. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimetry Can Be Used To Find Mark The Three That Are Correct Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. We do this using simple calorimetry. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. We can experimentally determine the relative. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5577734 Calorimetry Can Be Used To Find Mark The Three That Are Correct Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Ex phys ch 5 study. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Can Be Used To Find Mark The Three That Are Correct One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. We do this using simple calorimetry. Adding the enthalpy of reaction. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Can Be Used To Find Mark The Three That Are Correct Compare heat flow from hot to cold objects in an ideal. Apply the first law of thermodynamics to calorimetry. Which steps can be used to calculate the enthalpy of reaction iii correctly? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Define direct calorimetry and indirect calorimetry and describe how. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From users.highland.edu
Calorimetry Calorimetry Can Be Used To Find Mark The Three That Are Correct Apply the first law of thermodynamics to calorimetry. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Compare heat flow from hot to cold objects in an ideal. Which steps can be used to calculate the enthalpy of reaction iii correctly? Adding the enthalpy of reaction i and the enthalpy of reaction ii. For. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog Calorimetry Can Be Used To Find Mark The Three That Are Correct Apply the first law of thermodynamics to calorimetry. Which steps can be used to calculate the enthalpy of reaction iii correctly? Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. A calorimeter is. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.chegg.com
Calorimetry is a technique that can be used to Calorimetry Can Be Used To Find Mark The Three That Are Correct Adding the enthalpy of reaction i and the enthalpy of reaction ii. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Which steps can be used to calculate the enthalpy of reaction iii correctly? We can experimentally determine the relative amounts of energy released by a fuel. A calorimeter is a device. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimetry Can Be Used To Find Mark The Three That Are Correct For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Which steps can be used to calculate the enthalpy of reaction iii correctly? Compare heat flow from hot to cold objects in an ideal. Adding the enthalpy of reaction i and the enthalpy of reaction ii.. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimetry Can Be Used To Find Mark The Three That Are Correct One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Adding the enthalpy of reaction i and the enthalpy of reaction ii. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimetry Can Be Used To Find Mark The Three That Are Correct Apply the first law of thermodynamics to calorimetry. Adding the enthalpy of reaction i and the enthalpy of reaction ii. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimetry Can Be Used To Find Mark The Three That Are Correct Which steps can be used to calculate the enthalpy of reaction iii correctly? Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. Compare heat flow from. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From dxofzsmrb.blob.core.windows.net
Equation Used For Calorimetry at Devin Johnson blog Calorimetry Can Be Used To Find Mark The Three That Are Correct Adding the enthalpy of reaction i and the enthalpy of reaction ii. Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Ex. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Can Be Used To Find Mark The Three That Are Correct Apply the first law of thermodynamics to calorimetry. We do this using simple calorimetry. For example, when an exothermic. Define direct calorimetry and indirect calorimetry and describe how they are used to measure energy. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Where q is the amount of heat. Calorimetry Can Be Used To Find Mark The Three That Are Correct.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Can Be Used To Find Mark The Three That Are Correct We do this using simple calorimetry. Where q is the amount of heat (in joules), c is the heat capacity (in joules per degree celsius), and δ t is tfinal − tinitial (in degrees. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. One. Calorimetry Can Be Used To Find Mark The Three That Are Correct.