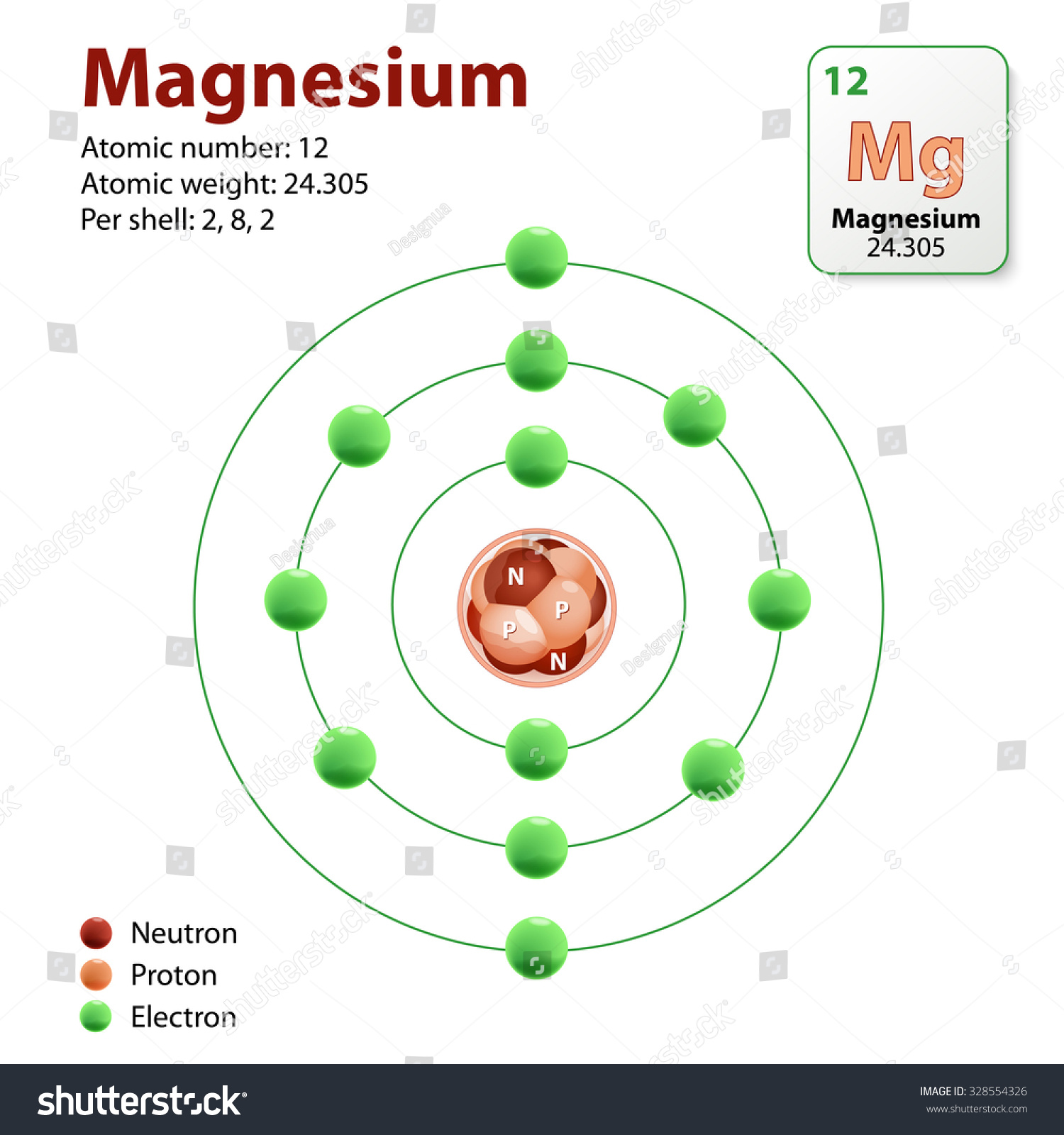
Magnesium Number Of Neutrons . The total number of protons and neutrons in an atom is called its mass number (a). Define isotope and mass number. Find out how to calculate the. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. The number of neutrons is therefore the. It has one isotope, 24 mg, and is essential for. Learn about the three main subatomic particles of atoms: Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Determine the number of protons, neutrons, and. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Describe the location, charge, and relative mass of the neutron. It has twelve protons and twelve neutrons.
from www.shutterstock.com
It has twelve protons and twelve neutrons. Determine the number of protons, neutrons, and. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. The number of neutrons is therefore the. Find out how to calculate the. It has one isotope, 24 mg, and is essential for. The total number of protons and neutrons in an atom is called its mass number (a). Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Learn about the three main subatomic particles of atoms: Define isotope and mass number.
Magnesium Atom. Diagram Representation Of The Element Magnesium. Neutrons, Protons And Electrons
Magnesium Number Of Neutrons Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Describe the location, charge, and relative mass of the neutron. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Learn about the three main subatomic particles of atoms: It has twelve protons and twelve neutrons. The total number of protons and neutrons in an atom is called its mass number (a). Find out how to calculate the. Define isotope and mass number. Determine the number of protons, neutrons, and. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. It has one isotope, 24 mg, and is essential for. The number of neutrons is therefore the. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12.
From www.science-revision.co.uk
Protons, neutrons and electrons. Magnesium Number Of Neutrons It has one isotope, 24 mg, and is essential for. Determine the number of protons, neutrons, and. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Find out how to calculate the. Learn about the three main subatomic particles of atoms: It has twelve protons and twelve neutrons. Magnesium is the. Magnesium Number Of Neutrons.
From www.slideserve.com
PPT Protons, Neutrons, Electrons PowerPoint Presentation, free download ID3287356 Magnesium Number Of Neutrons Describe the location, charge, and relative mass of the neutron. Define isotope and mass number. The total number of protons and neutrons in an atom is called its mass number (a). Find out how to calculate the. Determine the number of protons, neutrons, and. Magnesium is the 12th element in the periodic table and has a symbol of mg and. Magnesium Number Of Neutrons.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Number Of Neutrons Find out how to calculate the. Define isotope and mass number. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. It has one isotope, 24 mg, and is essential for. It has twelve protons and twelve neutrons. The total number of protons and neutrons in an atom is called. Magnesium Number Of Neutrons.
From guidemanualeruptivity.z14.web.core.windows.net
Magnesium Electron Configuration Diagram Magnesium Number Of Neutrons Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Determine the number of protons, neutrons, and. It has one isotope, 24 mg, and is essential for. Magnesium is the 12th element in. Magnesium Number Of Neutrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Number Of Neutrons Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. The total number of protons and neutrons in an atom is called its mass number (a). The number of neutrons is therefore the. It has one isotope, 24 mg, and is essential for. Magnesium is the 12th element in the periodic table. Magnesium Number Of Neutrons.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Number Of Neutrons Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. The total number of protons and neutrons in an atom is called its mass number (a). Find out how to calculate the. Determine the number of protons, neutrons, and. Define isotope and mass number. It has twelve protons and twelve neutrons. Learn. Magnesium Number Of Neutrons.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Number Of Neutrons It has twelve protons and twelve neutrons. The total number of protons and neutrons in an atom is called its mass number (a). Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic. Magnesium Number Of Neutrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Number Of Neutrons The total number of protons and neutrons in an atom is called its mass number (a). It has one isotope, 24 mg, and is essential for. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Magnesium is the 12th element in the periodic table and has a symbol of mg and. Magnesium Number Of Neutrons.
From www.numerade.com
SOLVEDGive complete symbols of each atom, including the atomic number and the mass number. a) a Magnesium Number Of Neutrons Learn about the three main subatomic particles of atoms: Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Magnesium is the 12th element in the periodic table and has a symbol of. Magnesium Number Of Neutrons.
From www.britannica.com
Magnesium Description, Properties, & Compounds Britannica Magnesium Number Of Neutrons It has twelve protons and twelve neutrons. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. It has one isotope, 24 mg, and is essential for. Find out how to calculate. Magnesium Number Of Neutrons.
From stock.adobe.com
Magnesium element with symbol Mg and atomic number 12.isolated molecular structure of atom on Magnesium Number Of Neutrons The total number of protons and neutrons in an atom is called its mass number (a). Describe the location, charge, and relative mass of the neutron. Determine the number of protons, neutrons, and. It has one isotope, 24 mg, and is essential for. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic. Magnesium Number Of Neutrons.
From slideplayer.com
Atomic Structure Chemistry. ppt download Magnesium Number Of Neutrons It has twelve protons and twelve neutrons. It has one isotope, 24 mg, and is essential for. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Determine the number of protons, neutrons, and. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic. Magnesium Number Of Neutrons.
From www.benjamin-mills.com
Electron arrangements Magnesium Number Of Neutrons The number of neutrons is therefore the. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. It has one isotope, 24 mg, and is essential for. Describe the location, charge,. Magnesium Number Of Neutrons.
From www.slideserve.com
PPT Nuclear model of atom PowerPoint Presentation ID6309354 Magnesium Number Of Neutrons Determine the number of protons, neutrons, and. The total number of protons and neutrons in an atom is called its mass number (a). It has twelve protons and twelve neutrons. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Describe the location, charge, and relative mass of the neutron. The number. Magnesium Number Of Neutrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Number Of Neutrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Learn about the three main subatomic particles of atoms: Define isotope and mass number. It has twelve protons and twelve neutrons. The total number of protons and neutrons in an atom is called its mass number (a). Magnesium is a. Magnesium Number Of Neutrons.
From material-properties.org
Magnesio Protones Neutrones Electrones Configuración electrónica Magnesium Number Of Neutrons Describe the location, charge, and relative mass of the neutron. Find out how to calculate the. Learn about the three main subatomic particles of atoms: Determine the number of protons, neutrons, and. The total number of protons and neutrons in an atom is called its mass number (a). It has one isotope, 24 mg, and is essential for. Magnesium is. Magnesium Number Of Neutrons.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium. Neutrons, Protons And Electrons Magnesium Number Of Neutrons Describe the location, charge, and relative mass of the neutron. Find out how to calculate the. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. It has one isotope, 24 mg, and is essential for. Determine the number of protons, neutrons, and. The total number of protons and neutrons in. Magnesium Number Of Neutrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Number Of Neutrons The total number of protons and neutrons in an atom is called its mass number (a). Define isotope and mass number. Learn about the three main subatomic particles of atoms: Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Describe the location, charge, and relative mass of the neutron. The number. Magnesium Number Of Neutrons.
From www.slideserve.com
PPT Part A Atomic Structure PowerPoint Presentation ID2589180 Magnesium Number Of Neutrons Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Describe the location, charge, and relative mass of the neutron. Determine the number of protons, neutrons, and. Define isotope and mass number. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12.. Magnesium Number Of Neutrons.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Magnesium Number Of Neutrons Describe the location, charge, and relative mass of the neutron. Find out how to calculate the. The total number of protons and neutrons in an atom is called its mass number (a). Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. It has one isotope, 24 mg, and is essential for.. Magnesium Number Of Neutrons.
From www.science-revision.co.uk
Protons, neutrons and electrons. Magnesium Number Of Neutrons Describe the location, charge, and relative mass of the neutron. Learn about the three main subatomic particles of atoms: Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Find out how to calculate the. The number of neutrons is therefore the. The total number of protons and neutrons in an atom. Magnesium Number Of Neutrons.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Periodic Table of the Elements Magnesium Number Of Neutrons Determine the number of protons, neutrons, and. It has twelve protons and twelve neutrons. Learn about the three main subatomic particles of atoms: The total number of protons and neutrons in an atom is called its mass number (a). The number of neutrons is therefore the. Describe the location, charge, and relative mass of the neutron. Magnesium is a chemical. Magnesium Number Of Neutrons.
From slideplayer.com
Unit 3 Nuclear Model of the atom ppt video online download Magnesium Number Of Neutrons Learn about the three main subatomic particles of atoms: It has one isotope, 24 mg, and is essential for. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Define isotope and mass number. Find out how to calculate the. Magnesium is a chemical element with atomic number 12 which means. Magnesium Number Of Neutrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Number Of Neutrons It has one isotope, 24 mg, and is essential for. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Find out how to calculate the. The number of neutrons is therefore the. Learn about the three main subatomic particles of atoms: The total number of protons and neutrons in an atom. Magnesium Number Of Neutrons.
From www.alamy.com
Magnesium atom diagram concept Stock Vector Image & Art Alamy Magnesium Number Of Neutrons Determine the number of protons, neutrons, and. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Learn about the three main subatomic particles of atoms: Find out how to calculate the.. Magnesium Number Of Neutrons.
From www.shutterstock.com
Magnesium Periodic Table Element Atomic Model Stock Vector (Royalty Free) 2184150887 Shutterstock Magnesium Number Of Neutrons Describe the location, charge, and relative mass of the neutron. The number of neutrons is therefore the. Define isotope and mass number. Determine the number of protons, neutrons, and. The total number of protons and neutrons in an atom is called its mass number (a). Find out how to calculate the. Magnesium is the 12th element in the periodic table. Magnesium Number Of Neutrons.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Magnesium Number Of Neutrons Describe the location, charge, and relative mass of the neutron. The number of neutrons is therefore the. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Learn about the three main subatomic particles of atoms: Magnesium is a chemical element with atomic number 12 which means there are 12 protons. Magnesium Number Of Neutrons.
From www.alamy.com
Symbol and electron diagram for Magnesium illustration Stock Vector Image & Art Alamy Magnesium Number Of Neutrons It has twelve protons and twelve neutrons. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Learn about the three main subatomic particles of atoms: Find out how to calculate the. The total number of protons and neutrons in an atom is called its mass number (a). Magnesium is the 12th. Magnesium Number Of Neutrons.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Protons are represented Magnesium Number Of Neutrons Learn about the three main subatomic particles of atoms: Determine the number of protons, neutrons, and. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. It has twelve protons and twelve neutrons. Describe the location, charge, and relative mass of the neutron. It has one isotope, 24 mg, and is essential. Magnesium Number Of Neutrons.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation, free download ID4271266 Magnesium Number Of Neutrons It has one isotope, 24 mg, and is essential for. Define isotope and mass number. Learn about the three main subatomic particles of atoms: Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number. Magnesium Number Of Neutrons.
From www.alamy.com
Atom Symbol for Magnesium Stock Vector Image & Art Alamy Magnesium Number Of Neutrons Find out how to calculate the. It has one isotope, 24 mg, and is essential for. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. The total number of protons and neutrons in an atom is called its mass number (a). Magnesium is a chemical element with atomic number. Magnesium Number Of Neutrons.
From www.numerade.com
SOLVED The number of protons and neutrons in an atom of magnesium25 is 1) 25 protons and 12 Magnesium Number Of Neutrons It has twelve protons and twelve neutrons. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. The total number of protons and neutrons in an atom is called its mass number (a). Define isotope and mass number. Magnesium is a chemical element with atomic number 12 which means there are. Magnesium Number Of Neutrons.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Number Of Neutrons It has twelve protons and twelve neutrons. Describe the location, charge, and relative mass of the neutron. It has one isotope, 24 mg, and is essential for. The number of neutrons is therefore the. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Magnesium is a chemical element with. Magnesium Number Of Neutrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Number Of Neutrons It has one isotope, 24 mg, and is essential for. Learn about the three main subatomic particles of atoms: Define isotope and mass number. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. Describe the location, charge, and relative mass of the neutron. The total number of protons and neutrons. Magnesium Number Of Neutrons.
From material-properties.org
Magnesium Protons Neutrons Electrons Electron Configuration Magnesium Number Of Neutrons Find out how to calculate the. Determine the number of protons, neutrons, and. It has twelve protons and twelve neutrons. Describe the location, charge, and relative mass of the neutron. Learn about the number of protons, neutrons, and electrons in magnesium, a shiny gray metal with atomic number 12. It has one isotope, 24 mg, and is essential for. Magnesium. Magnesium Number Of Neutrons.