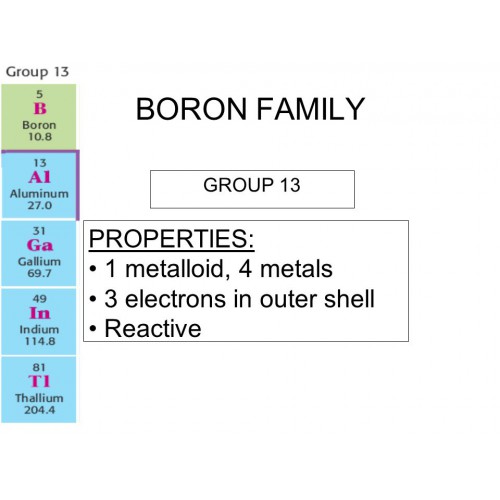
What Are The Properties Of Boron Group . Boron is capable of forming stable covalently bonded molecular networks. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Boron is the fifth element of the periodic table (z=5), located in group 13. Boron filaments have high strength, yet are lightweight. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. The energy band gap of elemental boron is 1.50. It is classified as a metalloid due it its properties. Pure crystalline boron is a black, lustrous semiconductor; Boron is unreactive towards oxygen in its crystalline form.
from proper-cooking.info
Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. It is classified as a metalloid due it its properties. Boron is capable of forming stable covalently bonded molecular networks. Boron is unreactive towards oxygen in its crystalline form. Boron filaments have high strength, yet are lightweight. Pure crystalline boron is a black, lustrous semiconductor; I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. The energy band gap of elemental boron is 1.50. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is the fifth element of the periodic table (z=5), located in group 13.
Physical Properties Of Boron
What Are The Properties Of Boron Group The energy band gap of elemental boron is 1.50. It is classified as a metalloid due it its properties. Boron filaments have high strength, yet are lightweight. Boron is the fifth element of the periodic table (z=5), located in group 13. Boron is unreactive towards oxygen in its crystalline form. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Pure crystalline boron is a black, lustrous semiconductor; Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Boron is capable of forming stable covalently bonded molecular networks. The energy band gap of elemental boron is 1.50. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures.
From studylib.net
GROUP 13 ELEMENTS THE BORON FAMILY What Are The Properties Of Boron Group Boron filaments have high strength, yet are lightweight. The energy band gap of elemental boron is 1.50. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Boron. What Are The Properties Of Boron Group.
From www.animalia-life.club
Physical Properties Of Boron What Are The Properties Of Boron Group The energy band gap of elemental boron is 1.50. Pure crystalline boron is a black, lustrous semiconductor; It is classified as a metalloid due it its properties. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3.. What Are The Properties Of Boron Group.
From www.britannica.com
Boron group element Properties & Facts Britannica What Are The Properties Of Boron Group Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. It is classified as a metalloid due it its properties. The energy band gap of elemental boron is 1.50. Boron is unreactive towards oxygen in its crystalline form. Pure crystalline boron is a black, lustrous semiconductor; Boron is capable of forming stable covalently bonded molecular. What Are The Properties Of Boron Group.
From eduinput.com
BoronDiscovery, Properties, And Applications What Are The Properties Of Boron Group The energy band gap of elemental boron is 1.50. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. I.e., it conducts electricity like a metal at high temperatures and is. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT Boron PowerPoint Presentation, free download ID5468516 What Are The Properties Of Boron Group The energy band gap of elemental boron is 1.50. Boron is the fifth element of the periodic table (z=5), located in group 13. Boron is capable of forming stable covalently bonded molecular networks. Pure crystalline boron is a black, lustrous semiconductor; I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Boron. What Are The Properties Of Boron Group.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table What Are The Properties Of Boron Group Boron filaments have high strength, yet are lightweight. Boron is unreactive towards oxygen in its crystalline form. It is classified as a metalloid due it its properties. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures.. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT ELEMENTS CHEMICAL & PHYSICAL PROPERTIES PowerPoint Presentation ID1784406 What Are The Properties Of Boron Group Boron is the fifth element of the periodic table (z=5), located in group 13. Boron is unreactive towards oxygen in its crystalline form. Pure crystalline boron is a black, lustrous semiconductor; The energy band gap of elemental boron is 1.50. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al,. What Are The Properties Of Boron Group.
From www.aakash.ac.in
Boron Family Properties, Occurrence & Anomalous Behaviour Chemistry Aakash AESL What Are The Properties Of Boron Group The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is the fifth element of the periodic table (z=5), located in group 13. Boron is capable of forming stable covalently bonded molecular networks. Finely divided amorphous boron reacts with oxygen on heating to form b. What Are The Properties Of Boron Group.
From mavink.com
Boron Family Periodic Table What Are The Properties Of Boron Group The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Boron is the fifth element of the periodic table (z=5), located in group 13. It is classified as. What Are The Properties Of Boron Group.
From www.vedantu.com
Boron Family Group 13 Elements General Properties of Boron family What Are The Properties Of Boron Group Pure crystalline boron is a black, lustrous semiconductor; Boron is capable of forming stable covalently bonded molecular networks. Boron is the fifth element of the periodic table (z=5), located in group 13. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Boron is unreactive towards oxygen in its crystalline form. Boron filaments have high. What Are The Properties Of Boron Group.
From www.animalia-life.club
Physical Properties Of Boron What Are The Properties Of Boron Group I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Boron is unreactive towards oxygen in its crystalline form. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). The energy band gap of elemental boron is 1.50.. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT THE ELEMENT FAMILIES PowerPoint Presentation, free download ID5194540 What Are The Properties Of Boron Group Boron is capable of forming stable covalently bonded molecular networks. It is classified as a metalloid due it its properties. The energy band gap of elemental boron is 1.50. Boron filaments have high strength, yet are lightweight. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Boron is the fifth element. What Are The Properties Of Boron Group.
From www.slideshare.net
The Periodic Table & Chemical Bonds What Are The Properties Of Boron Group The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron filaments have high strength, yet are lightweight. The energy band gap of elemental boron is 1.50. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Boron is the fifth. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT The representative Elements Groups 1A 4A PowerPoint Presentation ID6567283 What Are The Properties Of Boron Group Boron is the fifth element of the periodic table (z=5), located in group 13. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is capable of forming stable covalently bonded molecular networks. The energy band gap of elemental boron is 1.50. Pure crystalline boron. What Are The Properties Of Boron Group.
From classnotes.org.in
Physical Properties Of Boron Family Chemistry, Class 11, pBlock Elements What Are The Properties Of Boron Group I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Boron is capable of forming stable covalently bonded molecular networks. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. It is classified as a metalloid due it its properties. Boron is the fifth element of the. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT The Elements PowerPoint Presentation, free download ID5192313 What Are The Properties Of Boron Group Boron is the fifth element of the periodic table (z=5), located in group 13. The energy band gap of elemental boron is 1.50. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Pure crystalline boron is a black, lustrous semiconductor; Boron is capable of forming stable covalently bonded molecular networks. It. What Are The Properties Of Boron Group.
From utedzz.blogspot.com
Periodic Table Boron Family Periodic Table Timeline What Are The Properties Of Boron Group Pure crystalline boron is a black, lustrous semiconductor; The energy band gap of elemental boron is 1.50. It is classified as a metalloid due it its properties. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Boron filaments have high strength, yet are lightweight. I.e., it conducts electricity like a metal at high temperatures. What Are The Properties Of Boron Group.
From www.animalia-life.club
Physical Properties Of Boron What Are The Properties Of Boron Group Boron is capable of forming stable covalently bonded molecular networks. It is classified as a metalloid due it its properties. Pure crystalline boron is a black, lustrous semiconductor; The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). The energy band gap of elemental boron is. What Are The Properties Of Boron Group.
From www.britannica.com
Boron Properties, Uses, & Facts Britannica What Are The Properties Of Boron Group Boron filaments have high strength, yet are lightweight. Boron is capable of forming stable covalently bonded molecular networks. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Boron is the fifth element of the periodic table (z=5), located in group 13. The energy band gap of elemental boron is 1.50. Pure crystalline boron is. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT Properties of the Elements PowerPoint Presentation ID2204879 What Are The Properties Of Boron Group Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is capable of forming stable covalently bonded molecular networks. It is classified as a metalloid due it its properties. Boron. What Are The Properties Of Boron Group.
From ar.inspiredpencil.com
Boron Group On The Periodic Table What Are The Properties Of Boron Group The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is the fifth element of the periodic table (z=5), located in group 13. Boron is capable of forming stable covalently bonded molecular networks. Boron filaments have high strength, yet are lightweight. It is classified as. What Are The Properties Of Boron Group.
From www.animalia-life.club
Physical Properties Of Boron What Are The Properties Of Boron Group Boron is the fifth element of the periodic table (z=5), located in group 13. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Boron filaments have high strength, yet are lightweight. The energy band gap of elemental boron is 1.50. Boron is unreactive towards oxygen in its crystalline form. The elements present in group. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT Boron Family PowerPoint Presentation, free download ID2002494 What Are The Properties Of Boron Group Boron is unreactive towards oxygen in its crystalline form. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. Pure crystalline boron is a black, lustrous semiconductor; Boron filaments have high strength, yet are lightweight. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Boron is. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT Boron Family PowerPoint Presentation, free download ID2002494 What Are The Properties Of Boron Group Pure crystalline boron is a black, lustrous semiconductor; Boron is unreactive towards oxygen in its crystalline form. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron filaments have high strength, yet are lightweight. Boron is capable of forming stable covalently bonded molecular networks. Boron. What Are The Properties Of Boron Group.
From proper-cooking.info
Physical Properties Of Boron What Are The Properties Of Boron Group Boron is the fifth element of the periodic table (z=5), located in group 13. Boron is capable of forming stable covalently bonded molecular networks. It is classified as a metalloid due it its properties. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is. What Are The Properties Of Boron Group.
From chemwiki.ucdavis.edu
22.1 The Elements of Group 13 Chemwiki What Are The Properties Of Boron Group The energy band gap of elemental boron is 1.50. Pure crystalline boron is a black, lustrous semiconductor; Boron is the fifth element of the periodic table (z=5), located in group 13. Boron is unreactive towards oxygen in its crystalline form. It is classified as a metalloid due it its properties. Boron is capable of forming stable covalently bonded molecular networks.. What Are The Properties Of Boron Group.
From www.vectorstock.com
Diagram representation of the element boron Vector Image What Are The Properties Of Boron Group Boron is capable of forming stable covalently bonded molecular networks. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron filaments have high strength, yet are lightweight. The energy band gap of elemental boron is 1.50. Finely divided amorphous boron reacts with oxygen on heating. What Are The Properties Of Boron Group.
From klazskhpz.blob.core.windows.net
What Is Boron Used For Soil at Norma Bruhn blog What Are The Properties Of Boron Group Boron is capable of forming stable covalently bonded molecular networks. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Pure crystalline boron is a black, lustrous semiconductor;. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT Boron Group 3A PowerPoint Presentation, free download ID1415235 What Are The Properties Of Boron Group Boron is the fifth element of the periodic table (z=5), located in group 13. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3. The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is capable of forming stable covalently. What Are The Properties Of Boron Group.
From www.slideserve.com
PPT Boron PowerPoint Presentation, free download ID5468516 What Are The Properties Of Boron Group I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. It is classified as a metalloid due it its properties. Pure crystalline boron is a black, lustrous semiconductor; The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh).. What Are The Properties Of Boron Group.
From www.youtube.com
Occurrence and physical properties of Boron group pBlock ElementsI TN 12th Chemistry What Are The Properties Of Boron Group Pure crystalline boron is a black, lustrous semiconductor; Boron is the fifth element of the periodic table (z=5), located in group 13. It is classified as a metalloid due it its properties. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Finely divided amorphous boron reacts with oxygen on heating to. What Are The Properties Of Boron Group.
From scienceinfo.com
Boron (B) Element Occurrence, Properties, Uses, Toxicity What Are The Properties Of Boron Group The energy band gap of elemental boron is 1.50. Pure crystalline boron is a black, lustrous semiconductor; The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is capable of forming stable covalently bonded molecular networks. I.e., it conducts electricity like a metal at high. What Are The Properties Of Boron Group.
From www.britannica.com
Boron group element Trihalides, Properties, Uses Britannica What Are The Properties Of Boron Group The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is unreactive towards oxygen in its crystalline form. I.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. Pure crystalline boron is a black, lustrous semiconductor; Boron. What Are The Properties Of Boron Group.
From utedzz.blogspot.com
Boron Group In The Periodic Table Of Elements Periodic Table Timeline What Are The Properties Of Boron Group The elements present in group 13 of the modern periodic table are known as the boron family (includes b, al, ga, in, tl, nh). Boron is capable of forming stable covalently bonded molecular networks. Boron filaments have high strength, yet are lightweight. The energy band gap of elemental boron is 1.50. Boron is the fifth element of the periodic table. What Are The Properties Of Boron Group.
From www.animalia-life.club
Physical Properties Of Boron What Are The Properties Of Boron Group Boron is capable of forming stable covalently bonded molecular networks. It is classified as a metalloid due it its properties. Pure crystalline boron is a black, lustrous semiconductor; The energy band gap of elemental boron is 1.50. Boron filaments have high strength, yet are lightweight. Finely divided amorphous boron reacts with oxygen on heating to form b 2 o 3.. What Are The Properties Of Boron Group.