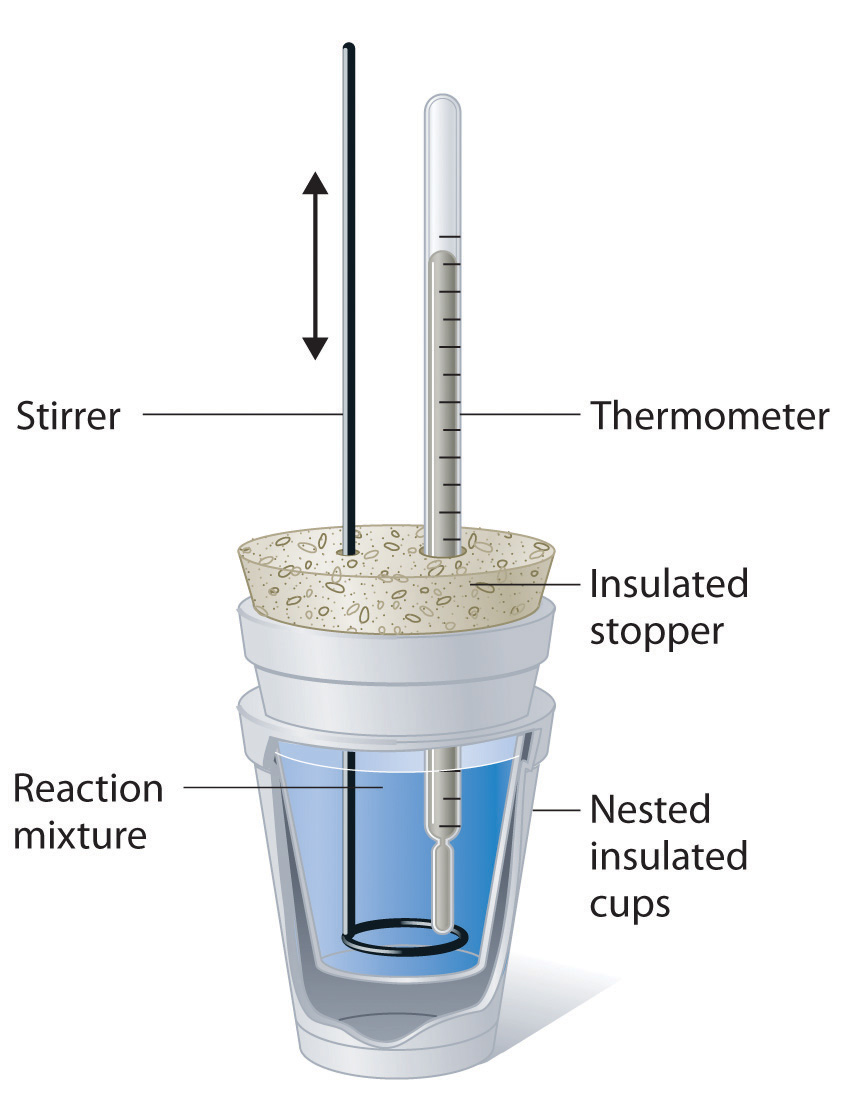
Calorimetry Of A Salt Solution Lab . The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. Learn how to measure heat and enthalpy changes using a calorimeter. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. As part of this lab, you will:
from users.highland.edu
The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. Learn how to measure heat and enthalpy changes using a calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. As part of this lab, you will: The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium.
Calorimetry
Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. Learn how to measure heat and enthalpy changes using a calorimeter. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. As part of this lab, you will: The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. As part of this. Calorimetry Of A Salt Solution Lab.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Of A Salt Solution Lab As part of this lab, you will: A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. Learn how to measure heat and enthalpy changes using a calorimeter. Learn how to use a parr. Calorimetry Of A Salt Solution Lab.
From www.youtube.com
Calorimetry Lab Heat of Solution of NaOH YouTube Calorimetry Of A Salt Solution Lab Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. Learn how to measure heat and enthalpy changes using a calorimeter. In calorimetry, an insulated reaction container, called a calorimeter,. Calorimetry Of A Salt Solution Lab.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool Calorimetry Of A Salt Solution Lab Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. Learn how. Calorimetry Of A Salt Solution Lab.
From animalia-life.club
Calorimeter Diagram Calorimetry Of A Salt Solution Lab The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat. Calorimetry Of A Salt Solution Lab.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. As part of this lab, you will: The purpose of this lab find the enthalpy of. Calorimetry Of A Salt Solution Lab.
From users.highland.edu
Calorimetry Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. Learn. Calorimetry Of A Salt Solution Lab.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimetry Of A Salt Solution Lab The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. Learn how to measure heat and enthalpy changes using a calorimeter. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. As part of this lab, you will: Learn how to use. Calorimetry Of A Salt Solution Lab.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Of A Salt Solution Lab The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. As part of this lab, you will: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device that is used to measure heat developed during a reaction. Calorimetry Of A Salt Solution Lab.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Of A Salt Solution Lab Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. Learn how to measure heat and enthalpy changes using a calorimeter. As part of this lab, you will: A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat. Calorimetry Of A Salt Solution Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Of A Salt Solution Lab This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. Learn how to measure heat and enthalpy changes using a calorimeter. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. As part of this lab, you will: The purpose of this lab is to use calorimetry. Calorimetry Of A Salt Solution Lab.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Calorimetry Of A Salt Solution Lab Learn how to measure heat and enthalpy changes using a calorimeter. As part of this lab, you will: Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat. Calorimetry Of A Salt Solution Lab.
From www.studocu.com
Unit 2 Lesson 2 Calorimetry of salt LAB Unit 2 Lesson 2 Calorimetry Calorimetry Of A Salt Solution Lab Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to. Calorimetry Of A Salt Solution Lab.
From www.chegg.com
Solved Lab Calorimetry of a salt solution Procedure Calorimetry Of A Salt Solution Lab A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. Learn how to measure heat and enthalpy changes using a calorimeter. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. Learn how to use a. Calorimetry Of A Salt Solution Lab.
From www.studocu.com
Chem Unit 2 Lab 2 Calorimetry Of A Salt Solution Lab Introduction Calorimetry Of A Salt Solution Lab A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. This. Calorimetry Of A Salt Solution Lab.
From www.youtube.com
Neutralization Calorimetry Examples YouTube Calorimetry Of A Salt Solution Lab The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. As part of this lab, you will: Learn how to measure heat and enthalpy changes using a calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with. Calorimetry Of A Salt Solution Lab.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Of A Salt Solution Lab As part of this lab, you will: Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. A calorimeter is a device that is used to measure heat developed during a reaction and. Calorimetry Of A Salt Solution Lab.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Of A Salt Solution Lab As part of this lab, you will: Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. Learn how to measure heat and enthalpy changes using a calorimeter. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat. Calorimetry Of A Salt Solution Lab.
From www.chegg.com
20.0589 Experiment as Report Sheet "Calorimetry c. Calorimetry Of A Salt Solution Lab As part of this lab, you will: This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. Learn how to measure heat and enthalpy changes using a calorimeter. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. Learn how to. Calorimetry Of A Salt Solution Lab.
From studylib.net
Lab Notes for Solution Calorimetry Calorimetry Of A Salt Solution Lab Learn how to measure heat and enthalpy changes using a calorimeter. Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. The purpose of this lab find. Calorimetry Of A Salt Solution Lab.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Of A Salt Solution Lab Learn how to measure heat and enthalpy changes using a calorimeter. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. Learn how to use a parr solution calorimeter to measure the. Calorimetry Of A Salt Solution Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Of A Salt Solution Lab The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. In calorimetry,. Calorimetry Of A Salt Solution Lab.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution. Calorimetry Of A Salt Solution Lab.
From www.semanticscholar.org
Figure 1 from High pressure differential scanning calorimetry of the Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. Learn how to measure heat and enthalpy changes using a calorimeter. The purpose of this. Calorimetry Of A Salt Solution Lab.
From chem.libretexts.org
5.6 Calorimetry Chemistry LibreTexts Calorimetry Of A Salt Solution Lab Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device that is used to measure heat developed during a reaction. Calorimetry Of A Salt Solution Lab.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Of A Salt Solution Lab The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions. Calorimetry Of A Salt Solution Lab.
From www.studocu.com
Calorimetry of a Salt Solution Lab Ali Sayed 201904 Mr. Lakhani Calorimetry Of A Salt Solution Lab Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. Learn how to measure heat and enthalpy changes using a calorimeter. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. This laboratory exercise aims to ascertain. Calorimetry Of A Salt Solution Lab.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Of A Salt Solution Lab As part of this lab, you will: The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. Learn how to measure heat and enthalpy changes using a calorimeter. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. Learn. Calorimetry Of A Salt Solution Lab.
From studylib.net
Lab 33 calorimetry lab Calorimetry Of A Salt Solution Lab Learn how to measure heat and enthalpy changes using a calorimeter. As part of this lab, you will: A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence. Calorimetry Of A Salt Solution Lab.
From courses.lumenlearning.com
Calorimetry General Chemistry Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. As part of this lab, you will: The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. The purpose of this lab is to use calorimetry to determine the enthalpy of a. Calorimetry Of A Salt Solution Lab.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Of A Salt Solution Lab The purpose of this lab find the enthalpy of two salts using a calorimetry experiment. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. As part of this lab, you will: A calorimeter is a device that is used to measure heat developed during a reaction. Calorimetry Of A Salt Solution Lab.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Calorimetry Of A Salt Solution Lab As part of this lab, you will: Learn how to use a parr solution calorimeter to measure the enthalpy change of the autoionization of water and its dependence on. The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. A calorimeter is a device that is used. Calorimetry Of A Salt Solution Lab.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Of A Salt Solution Lab The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. Learn how to measure heat and enthalpy changes using a calorimeter. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. Learn how to use a parr solution calorimeter. Calorimetry Of A Salt Solution Lab.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Of A Salt Solution Lab The purpose of this lab is to use calorimetry to determine the enthalpy of a solution for a salt to dissolve for sodium. As part of this lab, you will: A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the heat capacity. Learn how to measure heat and enthalpy changes using. Calorimetry Of A Salt Solution Lab.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Of A Salt Solution Lab In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. This laboratory exercise aims to ascertain the molar enthalpy of two distinct salt solutions by employing calorimetry techniques. A calorimeter is a device that is used to measure heat developed during a reaction and for calculating the. Calorimetry Of A Salt Solution Lab.