Bromine Chloride Dissociation . 357, i gave an account of prof. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Because chlorine is more reactive than bromine, it. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Bromine (i) chloride is a chloride of bromine. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. Meyer's remarkable observations on the density of chlorine at high. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6.
from www.youtube.com
When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Meyer's remarkable observations on the density of chlorine at high. Because chlorine is more reactive than bromine, it. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. 357, i gave an account of prof. Bromine (i) chloride is a chloride of bromine. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water.
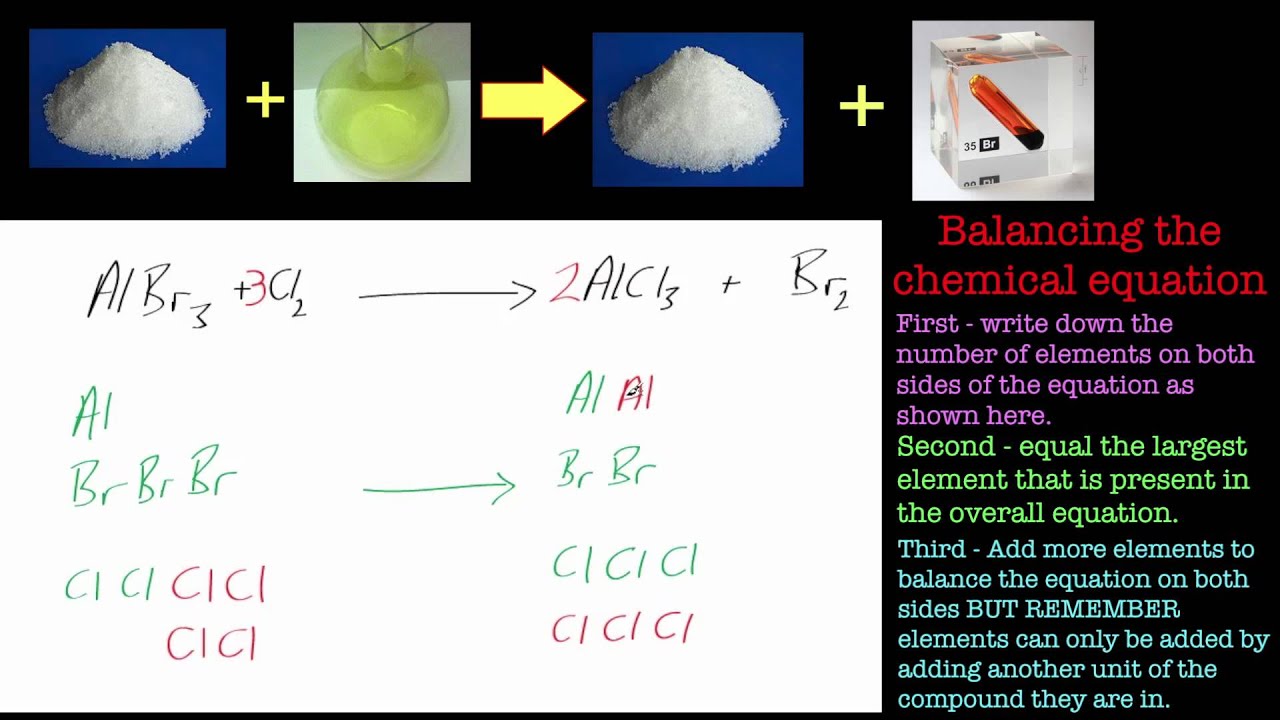
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine
Bromine Chloride Dissociation Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. Bromine (i) chloride is a chloride of bromine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Meyer's remarkable observations on the density of chlorine at high. 357, i gave an account of prof. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Because chlorine is more reactive than bromine, it.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Bromine Chloride Dissociation Bromine (i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution,. Bromine Chloride Dissociation.
From www.alamy.com
Bromine Chloride dangerous poisonous gas in chemical glassware Stock Bromine Chloride Dissociation Because chlorine is more reactive than bromine, it. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. 357, i gave an account of prof. Bromine (i) chloride is a chloride of bromine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes. Bromine Chloride Dissociation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine Chloride Dissociation 357, i gave an account of prof. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Meyer's remarkable observations on the density of chlorine at high. Bromine (i) chloride is a chloride of bromine. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. It is used as. Bromine Chloride Dissociation.
From www.youtube.com
Acid Dissociation Constant (Example) YouTube Bromine Chloride Dissociation Meyer's remarkable observations on the density of chlorine at high. Because chlorine is more reactive than bromine, it. 357, i gave an account of prof. Bromine (i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. The mixed halogen compounds readily hydrolyze (cotton and wilkinson. Bromine Chloride Dissociation.
From www.numerade.com
SOLVED QUESTION 3 Which alkyl halide would you expect to undergo SNI Bromine Chloride Dissociation 357, i gave an account of prof. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine chloride. Bromine Chloride Dissociation.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine Chloride Dissociation Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. Meyer's remarkable observations on the density of chlorine at high. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine (i) chloride is a chloride of bromine. When chlorine (as. Bromine Chloride Dissociation.
From en.ppt-online.org
Electrolysis online presentation Bromine Chloride Dissociation Meyer's remarkable observations on the density of chlorine at high. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine (i) chloride is a chloride of bromine. 357, i gave an account of prof. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). When chlorine (as a gas or dissolved. Bromine Chloride Dissociation.
From www.slideserve.com
PPT Chlorine Chemistry PowerPoint Presentation ID1910807 Bromine Chloride Dissociation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine (i) chloride is a chloride of bromine. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. When chlorine (as a gas or dissolved in water) is added to sodium. Bromine Chloride Dissociation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine Chloride Dissociation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. 357, i gave an account of prof. Because chlorine is more reactive than bromine, it. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Bromine (i) chloride is a chloride of bromine. It is used as. Bromine Chloride Dissociation.
From www.coursehero.com
[Solved] When chlorine gas is bubbled into a solution of sodium bromide Bromine Chloride Dissociation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. 357, i gave an account of prof. It is used as a biocide, specifically as an algaecide, fungicide,. Bromine Chloride Dissociation.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine Bromine Chloride Dissociation 357, i gave an account of prof. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. It. Bromine Chloride Dissociation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine Chloride Dissociation Because chlorine is more reactive than bromine, it. Bromine (i) chloride is a chloride of bromine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. The mixed halogen. Bromine Chloride Dissociation.
From www.youtube.com
Sodium bromide YouTube Bromine Chloride Dissociation Bromine (i) chloride is a chloride of bromine. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. It is used as a biocide, specifically as an algaecide, fungicide, and. Bromine Chloride Dissociation.
From www.chegg.com
Solved Use The Bond Dissociation Energies From The Table Bromine Chloride Dissociation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Meyer's remarkable observations on the density of chlorine at. Bromine Chloride Dissociation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Bromine Chloride Dissociation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. 357, i gave an account of prof. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. When chlorine (as a gas or dissolved in water) is added to sodium bromide. Bromine Chloride Dissociation.
From www.youtube.com
Chemistry Class 10 Electrolysis Electrolysis of molten lead bromide Bromine Chloride Dissociation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Because chlorine is more reactive than bromine, it. Meyer's remarkable observations on the density of chlorine at high. 357, i gave an account of prof. Bromine (i) chloride is a chloride of bromine. Bromine chloride and its. Bromine Chloride Dissociation.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1878282 Bromine Chloride Dissociation Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. Bromine (i) chloride is a chloride of bromine. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. Because chlorine is more reactive than bromine, it. When chlorine (as a. Bromine Chloride Dissociation.
From www.youtube.com
How to Balance KBr + Cl2 = KCl + Br2 (Potassium bromide + Chlorine gas Bromine Chloride Dissociation Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. Bromine (i) chloride is a chloride of bromine. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. 357, i gave an account of prof. It is used as a. Bromine Chloride Dissociation.
From www.numerade.com
SOLVED Write the electron transfer and ionic compound formation of the Bromine Chloride Dissociation Bromine (i) chloride is a chloride of bromine. Because chlorine is more reactive than bromine, it. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. When chlorine (as a. Bromine Chloride Dissociation.
From webmis.highland.cc.il.us
Lattice Energies in Ionic Solids Bromine Chloride Dissociation Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Because chlorine is more reactive than bromine, it. It is. Bromine Chloride Dissociation.
From www.numerade.com
SOLVED QUESTION 3 Which alkyl halide would you expect to undergo SNI Bromine Chloride Dissociation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine (i) chloride is a chloride of bromine. Because chlorine is more reactive than bromine, it. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Meyer's remarkable observations on the density of chlorine at high. Bromine chloride and its dissociation products. Bromine Chloride Dissociation.
From www.coursehero.com
Electrolysis Chemistry for Majors Atoms First Course Hero Bromine Chloride Dissociation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Because chlorine is more reactive than bromine, it. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of. Bromine Chloride Dissociation.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Chloride Dissociation Meyer's remarkable observations on the density of chlorine at high. Because chlorine is more reactive than bromine, it. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,.. Bromine Chloride Dissociation.
From insights.globalspec.com
Cooling Towers and the Fight Against Harmful Microbes Engineering360 Bromine Chloride Dissociation Bromine (i) chloride is a chloride of bromine. 357, i gave an account of prof. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Accordingly, bromine chloride. Bromine Chloride Dissociation.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Bromine Chloride Dissociation Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. 357, i gave an account of prof. Bromine (i) chloride is a chloride of bromine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Because chlorine is. Bromine Chloride Dissociation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine Chloride Dissociation Because chlorine is more reactive than bromine, it. Bromine (i) chloride is a chloride of bromine. Meyer's remarkable observations on the density of chlorine at high. 357, i gave an account of prof. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine. Bromine Chloride Dissociation.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Bromine Chloride Dissociation 357, i gave an account of prof. Meyer's remarkable observations on the density of chlorine at high. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Because chlorine is more reactive than bromine, it. Bromine (i) chloride is a chloride of bromine. Bromine chloride and its dissociation products may react with. Bromine Chloride Dissociation.
From www.youtube.com
Type of Reaction for NaBr + Cl2 = NaCl + Br2 YouTube Bromine Chloride Dissociation Because chlorine is more reactive than bromine, it. Meyer's remarkable observations on the density of chlorine at high. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Bromine (i) chloride is a chloride of bromine. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of. Bromine Chloride Dissociation.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry Bromine Chloride Dissociation Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. 357, i gave an account of prof. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of. Bromine Chloride Dissociation.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromine Chloride Dissociation 357, i gave an account of prof. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to. Bromine Chloride Dissociation.
From www.pinterest.com
Why benzyl chloride is highly reactive in SN1 reaction in spite of Bromine Chloride Dissociation Bromine (i) chloride is a chloride of bromine. Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Because chlorine is more reactive than bromine, it. The mixed halogen compounds readily hydrolyze (cotton. Bromine Chloride Dissociation.
From www.youtube.com
Chlorine Water + Sodium Bromide YouTube Bromine Chloride Dissociation The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. 357, i gave an account of prof. Bromine (i) chloride is a chloride of bromine. Because chlorine is more reactive than bromine, it. Meyer's remarkable observations on the density of chlorine at. Bromine Chloride Dissociation.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Bromine Chloride Dissociation Bromine (i) chloride is a chloride of bromine. Because chlorine is more reactive than bromine, it. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Bromine chloride and its dissociation products may react with water to form a variety of weak. Bromine Chloride Dissociation.
From brunofuga.adv.br
When Bromine Gas Reacts With Aqueous Sodium Hydroxide, The, 57 OFF Bromine Chloride Dissociation Meyer's remarkable observations on the density of chlorine at high. Bromine (i) chloride is a chloride of bromine. Because chlorine is more reactive than bromine, it. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Accordingly, bromine chloride dissociates in aqueous solution to bromine ions of positive i5 charge and to chloride ions of negative 6. Bromine chloride and. Bromine Chloride Dissociation.
From saylordotorg.github.io
Aqueous Solutions Bromine Chloride Dissociation Bromine chloride and its dissociation products may react with water to form a variety of weak and strong acids,. 357, i gave an account of prof. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. The mixed halogen compounds readily hydrolyze (cotton and wilkinson 1980). Because chlorine is more reactive than. Bromine Chloride Dissociation.