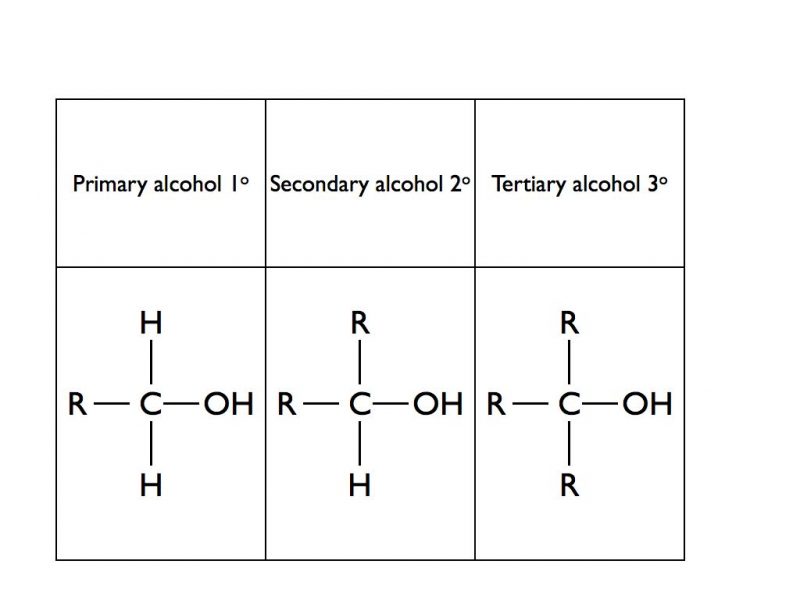
Distinguishing Test Between Alcohol And Phenol . ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. difference between alcohol and phenol now that we have learnt. When deprotonated, their conjugate bases. both alcohols and phenols are capable of acting as weakly acidic species; Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. several tests can be used for differentiating between an alcohol and phenol. Some of these are lucas test, ferric chloride test, sodium. Phenol being acidic turns blue litmus. Enols have a hydroxide bonded to an sp 2 carbon.
from www.pdfprof.com
When deprotonated, their conjugate bases. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. Phenol being acidic turns blue litmus. both alcohols and phenols are capable of acting as weakly acidic species; alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. difference between alcohol and phenol now that we have learnt. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. Enols have a hydroxide bonded to an sp 2 carbon.
chemical test to distinguish between alcohol and alkene
Distinguishing Test Between Alcohol And Phenol the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. Enols have a hydroxide bonded to an sp 2 carbon. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. difference between alcohol and phenol now that we have learnt. several tests can be used for differentiating between an alcohol and phenol. both alcohols and phenols are capable of acting as weakly acidic species; When deprotonated, their conjugate bases. alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. Phenol being acidic turns blue litmus. Some of these are lucas test, ferric chloride test, sodium. Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon.
From www.studypool.com
SOLUTION Chemistry difference between alcohol and phenol Studypool Distinguishing Test Between Alcohol And Phenol Enols have a hydroxide bonded to an sp 2 carbon. When deprotonated, their conjugate bases. Phenol being acidic turns blue litmus. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon. Distinguishing Test Between Alcohol And Phenol.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Distinguishing Test Between Alcohol And Phenol several tests can be used for differentiating between an alcohol and phenol. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. phenol is unsaturated aromatic alcohol, while. Distinguishing Test Between Alcohol And Phenol.
From brainly.in
Give a chemical test to distinguish between phenol and alcohol Brainly.in Distinguishing Test Between Alcohol And Phenol Enols have a hydroxide bonded to an sp 2 carbon. When deprotonated, their conjugate bases. difference between alcohol and phenol now that we have learnt. Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. several tests can be used for differentiating between an alcohol and phenol. phenol is unsaturated aromatic alcohol, while ethanol is saturated. Distinguishing Test Between Alcohol And Phenol.
From studylib.net
Experiment 8 properties of Alcohols and Phenols Distinguishing Test Between Alcohol And Phenol Phenol being acidic turns blue litmus. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. several tests can be used for differentiating between an alcohol and phenol. both alcohols and phenols are capable of acting as. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Alcohol Phenol Class 12 / Distinguish test / primary secondary tertiary Distinguishing Test Between Alcohol And Phenol ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: difference between alcohol and phenol now that we have learnt. Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. several tests can be used for differentiating between an alcohol and phenol. Enols have a. Distinguishing Test Between Alcohol And Phenol.
From www.pdfprof.com
chemical test to distinguish between alcohol and alkene Distinguishing Test Between Alcohol And Phenol Enols have a hydroxide bonded to an sp 2 carbon. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: When deprotonated, their conjugate bases. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. both alcohols and phenols are capable of acting as weakly. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Difference Between Alcohol and Phenol, Chemistry Lecture Sabaq.pk Distinguishing Test Between Alcohol And Phenol alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. When deprotonated, their conjugate bases. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: Phenol being acidic turns blue litmus. both alcohols and phenols are capable of. Distinguishing Test Between Alcohol And Phenol.
From studylib.net
Alcohols and Phenols Distinguishing Test Between Alcohol And Phenol difference between alcohol and phenol now that we have learnt. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: several tests can be used for differentiating between an alcohol and phenol. alcohols can undergo a wide variety of reactions, and because of this reactivity. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Distinguishing tests between 1,2,3 degree alcohol & Phenol 26 Part5 Distinguishing Test Between Alcohol And Phenol Some of these are lucas test, ferric chloride test, sodium. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: both alcohols and phenols are capable of acting as weakly acidic species; phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. several tests. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Distinction test for phenol and alcohol How to differentiate between Distinguishing Test Between Alcohol And Phenol Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols,. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Difference between phenols and alcohol hydroxy compounds cbse Distinguishing Test Between Alcohol And Phenol Some of these are lucas test, ferric chloride test, sodium. several tests can be used for differentiating between an alcohol and phenol. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. both alcohols and phenols are capable of acting as weakly acidic species; the key difference between alcohols and phenol is that the hydroxyl. Distinguishing Test Between Alcohol And Phenol.
From www.studypool.com
SOLUTION Alcohols_classification_reaction_phenol_reaction_quiz alcohol Distinguishing Test Between Alcohol And Phenol the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. Enols have a hydroxide bonded to an sp 2 carbon. alcohols can undergo a wide variety of reactions, and. Distinguishing Test Between Alcohol And Phenol.
From www.pdfprof.com
chemical test to distinguish between alcohol and alkene Distinguishing Test Between Alcohol And Phenol Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. When deprotonated, their conjugate bases. ethanol (c 2. Distinguishing Test Between Alcohol And Phenol.
From collegedunia.com
Nomenclature of Alcohols Phenols and Ethers Rules and Examples Distinguishing Test Between Alcohol And Phenol both alcohols and phenols are capable of acting as weakly acidic species; difference between alcohol and phenol now that we have learnt. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to. Distinguishing Test Between Alcohol And Phenol.
From askfilo.com
Q. 6. Write any four differences between phenols and alcohols. Filo Distinguishing Test Between Alcohol And Phenol alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: When deprotonated, their conjugate bases. difference between alcohol and phenol now that we have learnt. several tests. Distinguishing Test Between Alcohol And Phenol.
From askfilo.com
related to phenol.Ans. Differences between Phenol and Alcohol (M.P. 20.. Distinguishing Test Between Alcohol And Phenol ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: Phenol being acidic turns blue litmus. both alcohols and phenols are capable of acting as weakly acidic species; Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. the key difference between alcohols and phenol. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
difference between alcohol and phenol alcohol vs phenol chemistry Distinguishing Test Between Alcohol And Phenol alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. Some of these are lucas test, ferric chloride test, sodium. the key difference between alcohols and phenol. Distinguishing Test Between Alcohol And Phenol.
From www.studypool.com
SOLUTION Alcohols_classification_reaction_phenol_reaction_quiz alcohol Distinguishing Test Between Alcohol And Phenol Phenol being acidic turns blue litmus. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. Some of these are lucas test, ferric chloride test, sodium. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: the key difference between alcohols and phenol is that. Distinguishing Test Between Alcohol And Phenol.
From www.toppr.com
Give a chemical test to distinguish betweenphenol and benzyl Distinguishing Test Between Alcohol And Phenol both alcohols and phenols are capable of acting as weakly acidic species; Enols have a hydroxide bonded to an sp 2 carbon. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a. Distinguishing Test Between Alcohol And Phenol.
From thecontentauthority.com
Alcohol vs Phenol Meaning And Differences Distinguishing Test Between Alcohol And Phenol several tests can be used for differentiating between an alcohol and phenol. When deprotonated, their conjugate bases. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: Phenol being acidic turns blue litmus. alcohols can undergo a wide variety of reactions, and because of this reactivity. Distinguishing Test Between Alcohol And Phenol.
From dxoyhnufj.blob.core.windows.net
How Are Phenols And Alcohols Similar at Erin Blair blog Distinguishing Test Between Alcohol And Phenol difference between alcohol and phenol now that we have learnt. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. Phenol being acidic turns blue litmus. Alcohols have. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Distinction between Alcohol and Phenol/ Uses of Phenol / AJT Chemistry Distinguishing Test Between Alcohol And Phenol ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. difference between alcohol and phenol now that we have learnt. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. the key difference. Distinguishing Test Between Alcohol And Phenol.
From pediaa.com
Difference Between Alcohol and Phenol Definition, Structure, Use Distinguishing Test Between Alcohol And Phenol Some of these are lucas test, ferric chloride test, sodium. alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. Enols have a hydroxide bonded to an sp 2 carbon. several tests can be used for differentiating between an alcohol and phenol. ethanol (c 2 h 5 oh) and. Distinguishing Test Between Alcohol And Phenol.
From www.studypool.com
SOLUTION Chemistry difference between alcohol and phenol Studypool Distinguishing Test Between Alcohol And Phenol When deprotonated, their conjugate bases. several tests can be used for differentiating between an alcohol and phenol. both alcohols and phenols are capable of acting as weakly acidic species; Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can. Distinguishing Test Between Alcohol And Phenol.
From www.meritnation.com
Pls let me know all the distinguish test and when to use them Form the Distinguishing Test Between Alcohol And Phenol ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: several tests can be used for differentiating between an alcohol and phenol. both alcohols and phenols are capable of acting as weakly acidic species; Enols have a hydroxide bonded to an sp 2 carbon. When deprotonated,. Distinguishing Test Between Alcohol And Phenol.
From www.differencebetween.com
Difference Between Alcohols and Phenols Compare the Difference Distinguishing Test Between Alcohol And Phenol Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. difference between alcohol and phenol now that we have learnt. When deprotonated, their conjugate bases. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh). Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Difference Between Alcohol And Phenol?Class Series YouTube Distinguishing Test Between Alcohol And Phenol difference between alcohol and phenol now that we have learnt. Enols have a hydroxide bonded to an sp 2 carbon. ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: alcohols can undergo a wide variety of reactions, and because of this reactivity and because they. Distinguishing Test Between Alcohol And Phenol.
From pediaa.com
Difference Between Alcohol and Phenol Definition, Structure, Use Distinguishing Test Between Alcohol And Phenol Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. several tests can be used for differentiating between. Distinguishing Test Between Alcohol And Phenol.
From pdfslide.net
(PDF) PHENOLS GneetTESTS TO DISTINGUISH BETWEEN ALCOHOLS AND PHENOLS Distinguishing Test Between Alcohol And Phenol ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: difference between alcohol and phenol now that we have learnt. the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Alcohol, Phenol & Ether 10 Iodoform test Chemical Test of alcohol Distinguishing Test Between Alcohol And Phenol ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. several tests can be used for differentiating between an alcohol and phenol. both alcohols and phenols are. Distinguishing Test Between Alcohol And Phenol.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Distinguishing Test Between Alcohol And Phenol Some of these are lucas test, ferric chloride test, sodium. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. difference between alcohol and phenol now that we have learnt. Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. several tests can be used for differentiating between an alcohol and phenol. When deprotonated, their. Distinguishing Test Between Alcohol And Phenol.
From www.slideserve.com
PPT PHENOL PowerPoint Presentation, free download ID1818685 Distinguishing Test Between Alcohol And Phenol difference between alcohol and phenol now that we have learnt. phenol is unsaturated aromatic alcohol, while ethanol is saturated aliphatic alcohol. Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. Enols have a hydroxide bonded to an sp 2 carbon. When deprotonated, their conjugate bases. the key difference between alcohols and phenol is that the. Distinguishing Test Between Alcohol And Phenol.
From www.youtube.com
Distinguish Between Alcohol And Phenol Chemical Test Of Phenol Distinguishing Test Between Alcohol And Phenol the key difference between alcohols and phenol is that the hydroxyl group of phenol is bonded directly to a carbon atom of an aromatic ring, whereas in other alcohols, the hydroxyl group is bonded to a saturated carbon atom. Enols have a hydroxide bonded to an sp 2 carbon. both alcohols and phenols are capable of acting as. Distinguishing Test Between Alcohol And Phenol.
From www.pw.live
Difference Between Alcohol And Phenol Distinguishing Test Between Alcohol And Phenol Enols have a hydroxide bonded to an sp 2 carbon. Alcohols have hydroxide groups (oh) bonded to an sp 3 carbon. Phenol being acidic turns blue litmus. difference between alcohol and phenol now that we have learnt. both alcohols and phenols are capable of acting as weakly acidic species; ethanol (c 2 h 5 oh) and phenol. Distinguishing Test Between Alcohol And Phenol.
From www.geeksforgeeks.org
Nomenclature of Alcohols, Phenols and Ethers Rules and Examples Distinguishing Test Between Alcohol And Phenol ethanol (c 2 h 5 oh) and phenol (c 6 h 5 oh) may be distinguished by the following four tests: alcohols can undergo a wide variety of reactions, and because of this reactivity and because they can be. Phenol being acidic turns blue litmus. Enols have a hydroxide bonded to an sp 2 carbon. the key. Distinguishing Test Between Alcohol And Phenol.