Class 1 Medical Device Examples Uk . The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Syringes without needles, medicine spoons, spectacle. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. The udi system provides a consistent and standard way to identify medical devices throughout their. Examples of classification are given below: This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes:
from www.ce-marking.com
We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: Examples of classification are given below: The udi system provides a consistent and standard way to identify medical devices throughout their. Syringes without needles, medicine spoons, spectacle. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market.
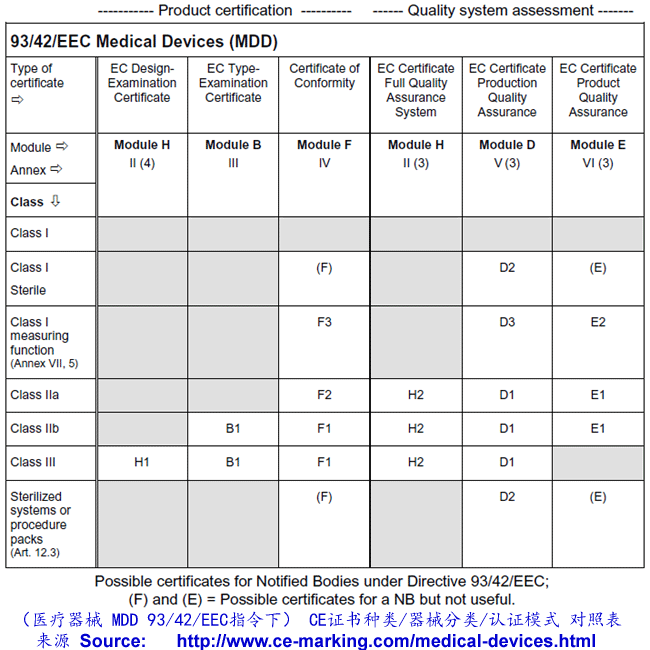
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark
Class 1 Medical Device Examples Uk Syringes without needles, medicine spoons, spectacle. Syringes without needles, medicine spoons, spectacle. The udi system provides a consistent and standard way to identify medical devices throughout their. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. Examples of classification are given below: This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes:
From www.linkedin.com
MDR IMPLEMENTATION GUIDE FOR CLASS 1 MEDICAL DEVICES Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1. Class 1 Medical Device Examples Uk.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Class 1 Medical Device Examples Uk This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: Syringes without needles, medicine spoons, spectacle. The medical device coordination group (mdcg) has drafted a guidance document that describes. Class 1 Medical Device Examples Uk.
From angelanjohnson.com
Medical Devices Angela N Johnson Class 1 Medical Device Examples Uk The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. Examples of classification are given below: This states that ‘medical. Class 1 Medical Device Examples Uk.
From www.devicelab.com
FDA Class 1 Medical Device Overview DeviceLab Class 1 Medical Device Examples Uk This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: We can’t list all the different types of devices but the examples below give an idea of the range. Class 1 Medical Device Examples Uk.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Syringes without needles, medicine spoons, spectacle. Examples of classification are given below: We can’t list all the different types of. Class 1 Medical Device Examples Uk.
From medium.com
The 3 FDA medical device classes [differences and examples explained Class 1 Medical Device Examples Uk The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Examples of classification are given below: The udi system provides a consistent and standard way to identify medical devices throughout their. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material,. Class 1 Medical Device Examples Uk.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Class 1 Medical Device Examples Uk The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. The udi system provides a consistent and standard way to. Class 1 Medical Device Examples Uk.
From mavink.com
Types Of Medical Devices List Class 1 Medical Device Examples Uk Syringes without needles, medicine spoons, spectacle. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: The udi system provides a consistent and standard way to identify medical devices. Class 1 Medical Device Examples Uk.
From catalogue.brunswickfyr.ca
First Aid Kit, Class 1 Medical Device, Plastic Box Brunswick Fyr & Safety Class 1 Medical Device Examples Uk The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Examples of classification are given below: Syringes without needles, medicine spoons, spectacle. The udi system provides a consistent and standard way to identify medical devices throughout their. We can’t list all the different types of. Class 1 Medical Device Examples Uk.
From www.i3cglobal.com
Class 1 Medical Device with CE Conformity Assessment Route Class 1 Medical Device Examples Uk Syringes without needles, medicine spoons, spectacle. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for. Class 1 Medical Device Examples Uk.
From www.presentationeze.com
Class 1 Medical Device PresentationEZE Class 1 Medical Device Examples Uk We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. Syringes without needles, medicine spoons, spectacle. The udi system provides a consistent and standard way to identify medical devices throughout their. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material,. Class 1 Medical Device Examples Uk.
From www.scnindustrial.com
SAFECROSS Office Standard First Aid Kits, Class 1 Medical Device, Metal Class 1 Medical Device Examples Uk We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. The udi system provides a consistent and standard way to identify medical devices throughout their. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1. Class 1 Medical Device Examples Uk.
From issuu.com
Class 1 medical device by David Waya Issuu Class 1 Medical Device Examples Uk Examples of classification are given below: This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: We can’t list all the different types of devices but the examples below. Class 1 Medical Device Examples Uk.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Class 1 Medical Device Examples Uk Syringes without needles, medicine spoons, spectacle. Examples of classification are given below: The udi system provides a consistent and standard way to identify medical devices throughout their. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. This states that ‘medical device’ means “any instrument,. Class 1 Medical Device Examples Uk.
From cortex-design.com
Cortex Design • What's My FDA Medical Device Classification? Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the. Class 1 Medical Device Examples Uk.
From medicaldevicehq.com
Different classifications rules for medical device software An Class 1 Medical Device Examples Uk Syringes without needles, medicine spoons, spectacle. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: The udi system provides a consistent and standard way to identify medical devices. Class 1 Medical Device Examples Uk.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Class 1 Medical Device Examples Uk This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: The udi system provides a consistent and standard way to identify medical devices throughout their. Syringes without needles, medicine. Class 1 Medical Device Examples Uk.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Class 1 Medical Device Examples Uk Examples of classification are given below: We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. The udi system provides. Class 1 Medical Device Examples Uk.
From www.arenasolutions.com
Class I Device Definition Arena Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the. Class 1 Medical Device Examples Uk.
From www.regdesk.co
What Is A Class 1 Medical Device RegDesk Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Syringes without needles, medicine spoons, spectacle. Examples of classification are given below: We can’t list all the different types of. Class 1 Medical Device Examples Uk.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: We can’t list all. Class 1 Medical Device Examples Uk.
From exyalhmkk.blob.core.windows.net
Medical Instruments Classification at Jane Drakeford blog Class 1 Medical Device Examples Uk The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. The udi system provides a consistent and standard way to identify medical devices throughout their. Examples of classification are given below: Syringes without needles, medicine spoons, spectacle. We can’t list all the different types of. Class 1 Medical Device Examples Uk.
From lifechanginginnovation.org
What is a Medical Device? Life Changing Innovation Class 1 Medical Device Examples Uk This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: We can’t list all the different types of devices but the examples below give an idea of the range. Class 1 Medical Device Examples Uk.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Class 1 Medical Device Examples Uk Examples of classification are given below: The udi system provides a consistent and standard way to identify medical devices throughout their. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. We can’t list all the different types of devices but the examples below give. Class 1 Medical Device Examples Uk.
From www.slideshare.net
Class 1 medical device classification Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. Examples of classification are given below: Syringes without needles, medicine spoons, spectacle. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one. Class 1 Medical Device Examples Uk.
From www.regdesk.co
What Is A Class 1 Medical Device RegDesk Class 1 Medical Device Examples Uk The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Syringes without needles, medicine spoons, spectacle. Examples of classification are given below: The udi system provides a consistent and standard way to identify medical devices throughout their. This states that ‘medical device’ means “any instrument,. Class 1 Medical Device Examples Uk.
From www.greenlight.guru
What is an FDA Class 1 Medical Device? [+Examples] Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. Examples of classification are given below: The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Syringes without needles, medicine spoons, spectacle. We can’t list all the different types of. Class 1 Medical Device Examples Uk.
From qualitysmartsolutions.com
Understanding Class 1 Medical Devices Approval & Importance Class 1 Medical Device Examples Uk Examples of classification are given below: Syringes without needles, medicine spoons, spectacle. We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. The udi system provides a consistent and standard way to identify medical devices throughout their. The medical device coordination group (mdcg) has drafted. Class 1 Medical Device Examples Uk.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Class 1 Medical Device Examples Uk This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: Syringes without needles, medicine spoons, spectacle. The medical device coordination group (mdcg) has drafted a guidance document that describes. Class 1 Medical Device Examples Uk.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Class 1 Medical Device Examples Uk The udi system provides a consistent and standard way to identify medical devices throughout their. This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: The medical device coordination. Class 1 Medical Device Examples Uk.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Class 1 Medical Device Examples Uk Examples of classification are given below: Syringes without needles, medicine spoons, spectacle. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. The udi system provides a consistent and standard way to identify medical devices throughout their. This states that ‘medical device’ means “any instrument,. Class 1 Medical Device Examples Uk.
From www.greenlight.guru
What is an FDA Class 1 Medical Device? [+Examples] Class 1 Medical Device Examples Uk This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class. Class 1 Medical Device Examples Uk.
From simplerqms.com
EU MDR Medical Device Classification Classes and Examples Class 1 Medical Device Examples Uk We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. The medical device coordination group (mdcg) has drafted a guidance document that describes how manufacturers should place their class 1 medical devices on the market. Syringes without needles, medicine spoons, spectacle. This states that ‘medical. Class 1 Medical Device Examples Uk.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Class 1 Medical Device Examples Uk This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: The udi system provides a consistent and standard way to identify medical devices throughout their. The medical device coordination. Class 1 Medical Device Examples Uk.
From laegemiddelstyrelsen.dk
Medical devices Class 1 Medical Device Examples Uk We can’t list all the different types of devices but the examples below give an idea of the range of products that are medical devices. Syringes without needles, medicine spoons, spectacle. Examples of classification are given below: This states that ‘medical device’ means “any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be. Class 1 Medical Device Examples Uk.