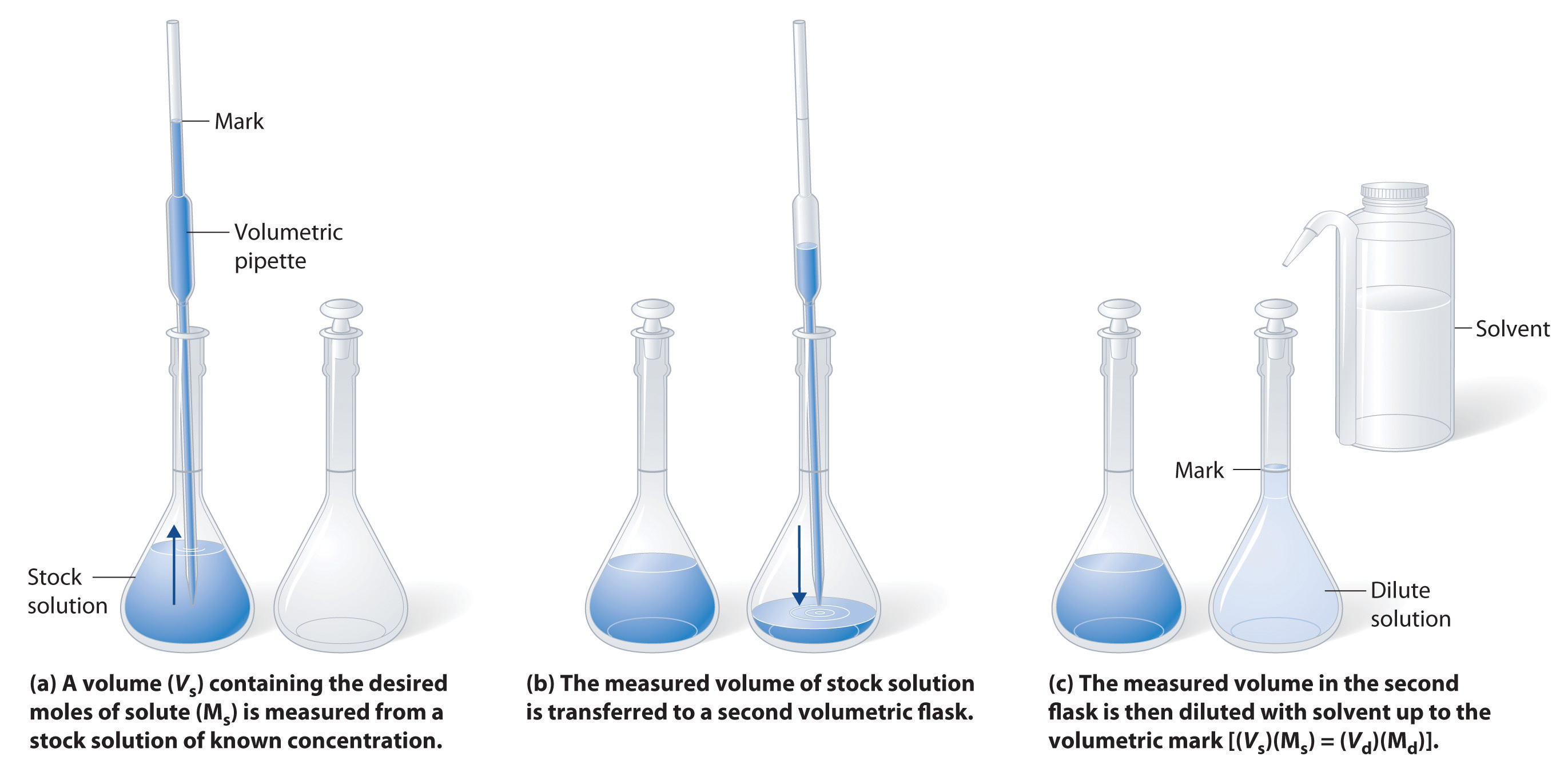
Dilutions Definition Chemistry . In a level chemistry, dilution only occurs with water. You can add water to. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. A dilute solution is one. The amount of water added to a solute will change its concentration. Dilution is when you add a solvent to a solution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent.
from chem.libretexts.org
Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In a level chemistry, dilution only occurs with water. Concentration is the removal of solvent, which increases the concentration of. A dilute solution is one. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. We will begin our discussion of solution concentration with two related and relative terms: The amount of water added to a solute will change its concentration. Dilution is when you add a solvent to a solution.
5.2 Solutions and Dilutions Chemistry LibreTexts
Dilutions Definition Chemistry Dilution is when you add a solvent to a solution. You can add water to. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our discussion of solution concentration with two related and relative terms: Dilution is when you add a solvent to a solution. Concentration is the removal of solvent, which increases the concentration of. A dilute solution is one. In a level chemistry, dilution only occurs with water. The amount of water added to a solute will change its concentration.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilutions Definition Chemistry Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. The amount of water added. Dilutions Definition Chemistry.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilutions Definition Chemistry We will begin our discussion of solution concentration with two related and relative terms: The amount of water added to a solute will change its concentration. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is when you add a solvent to a solution. In both dilution and. Dilutions Definition Chemistry.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilutions Definition Chemistry Dilution is when you add a solvent to a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is the process of reducing the concentration of a solute in solution,. Dilutions Definition Chemistry.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilutions Definition Chemistry Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. The amount of water added to a solute will change its concentration. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. In a level. Dilutions Definition Chemistry.
From klaivqdcu.blob.core.windows.net
What Does Dilute In Chemistry Mean at Sharron Wallace blog Dilutions Definition Chemistry Dilution is when you add a solvent to a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is the process of. Dilutions Definition Chemistry.
From www.youtube.com
VA06 Dilution and Aliquot Calculations Year 12 WACE Chemistry YouTube Dilutions Definition Chemistry In a level chemistry, dilution only occurs with water. Dilution is when you add a solvent to a solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the addition of solvent, which decreases the concentration. Dilutions Definition Chemistry.
From ceedzojh.blob.core.windows.net
Dilution Host Definition at Dana Chubb blog Dilutions Definition Chemistry Concentration is the removal of solvent, which increases the concentration of. We will begin our discussion of solution concentration with two related and relative terms: Dilution is when you add a solvent to a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing the concentration of. Dilutions Definition Chemistry.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilutions Definition Chemistry Dilution is when you add a solvent to a solution. You can add water to. Concentration is the removal of solvent, which increases the concentration of. A dilute solution is one. The amount of water added to a solute will change its concentration. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing. Dilutions Definition Chemistry.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilutions Definition Chemistry We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. You can add water to. In a level chemistry, dilution only occurs with water. Dilution is when you add a solvent to a solution. Dilution. Dilutions Definition Chemistry.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilutions Definition Chemistry Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Concentration is the removal. Dilutions Definition Chemistry.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilutions Definition Chemistry A dilute solution is one. In a level chemistry, dilution only occurs with water. In both dilution and concentration,. The amount of water added to a solute will change its concentration. You can add water to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases. Dilutions Definition Chemistry.
From klakbhtvf.blob.core.windows.net
Dilute Solution Has What at Virginia Sebastian blog Dilutions Definition Chemistry Dilution is when you add a solvent to a solution. The amount of water added to a solute will change its concentration. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. In both dilution and concentration,. Dilution is the process of “lowering the concentration of a solute in a solution. Dilutions Definition Chemistry.
From dxogrtxrg.blob.core.windows.net
Dilution Example In Chemistry at Ignacio Alvarez blog Dilutions Definition Chemistry The amount of water added to a solute will change its concentration. Dilution is when you add a solvent to a solution. A dilute solution is one. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. In both dilution and concentration,. Concentration is the removal of solvent, which increases. Dilutions Definition Chemistry.
From dxoecpgvz.blob.core.windows.net
How To Dilutions at Christopher Stevenson blog Dilutions Definition Chemistry You can add water to. Concentration is the removal of solvent, which increases the concentration of. Dilution is when you add a solvent to a solution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. In both dilution and concentration,. Dilution is the process of “lowering the concentration of. Dilutions Definition Chemistry.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Dilutions Definition Chemistry Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our discussion of solution concentration. Dilutions Definition Chemistry.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilutions Definition Chemistry We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of solvent, which increases the concentration of. The amount of water added to a solute will change its concentration. A dilute solution is one. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In a. Dilutions Definition Chemistry.
From facts.net
13 Surprising Facts About Dilution Dilutions Definition Chemistry Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In a level chemistry, dilution only occurs with water. The amount of water added to a solute will change its concentration. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is when. Dilutions Definition Chemistry.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe Dilutions Definition Chemistry Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Concentration is the removal of solvent, which increases the concentration of. Dilution is when you add a solvent to a solution. In a level chemistry, dilution only occurs with water. We will begin our discussion of. Dilutions Definition Chemistry.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilutions Definition Chemistry Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. You can add water to. In a level chemistry, dilution only occurs with water. Dilution is. Dilutions Definition Chemistry.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilutions Definition Chemistry In both dilution and concentration,. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. A dilute solution is one. In a level chemistry, dilution only occurs with water. Dilution is when you add a solvent to a solution. The amount of water added to a solute will change its. Dilutions Definition Chemistry.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilutions Definition Chemistry We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is when you add a solvent to a solution. In both dilution and concentration,. The amount of water added to a solute will change its concentration. In a level. Dilutions Definition Chemistry.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilutions Definition Chemistry Dilution is when you add a solvent to a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The amount of water added to a solute will change its concentration. A. Dilutions Definition Chemistry.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation ID6829039 Dilutions Definition Chemistry Concentration is the removal of solvent, which increases the concentration of. Dilution is when you add a solvent to a solution. You can add water to. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. A dilute solution is one. In both dilution and concentration,.. Dilutions Definition Chemistry.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilutions Definition Chemistry A dilute solution is one. Concentration is the removal of solvent, which increases the concentration of. In a level chemistry, dilution only occurs with water. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is when you add a solvent to a solution. In both dilution and concentration,. Dilution is the process. Dilutions Definition Chemistry.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilutions Definition Chemistry In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. Dilution is when you add a solvent to a solution. In a level chemistry, dilution only occurs with water. We will begin our discussion of solution concentration with two related and relative terms: The. Dilutions Definition Chemistry.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilutions Definition Chemistry You can add water to. We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the. Dilutions Definition Chemistry.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilutions Definition Chemistry Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is when you add a solvent to a solution. The amount of water added to a solute will change. Dilutions Definition Chemistry.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilutions Definition Chemistry Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You can add water to. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution. Dilutions Definition Chemistry.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilutions Definition Chemistry Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. A dilute solution is one. You can add water to. Dilution is when you add a solvent to a solution. In both dilution and concentration,. In a level chemistry, dilution only occurs with water. Concentration is the removal of solvent,. Dilutions Definition Chemistry.
From ceedzojh.blob.core.windows.net
Dilution Host Definition at Dana Chubb blog Dilutions Definition Chemistry Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is when you add a solvent to a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing the concentration of a. Dilutions Definition Chemistry.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilutions Definition Chemistry Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. We will begin our discussion of solution concentration with two related and relative terms: Dilution is. Dilutions Definition Chemistry.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilutions Definition Chemistry Concentration is the removal of solvent, which increases the concentration of. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. In a level chemistry, dilution only occurs with water. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our. Dilutions Definition Chemistry.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilutions Definition Chemistry Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In a level chemistry, dilution only occurs with water. In both dilution and concentration,. Concentration is the removal of solvent, which increases the concentration. Dilutions Definition Chemistry.
From www.studypool.com
SOLUTION Dilution chemistry Studypool Dilutions Definition Chemistry Dilution is when you add a solvent to a solution. You can add water to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of “lowering the concentration of a solute in a. Dilutions Definition Chemistry.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Overview ( Video ) Chemistry CK12 Dilutions Definition Chemistry We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The amount of water added to a solute will change its concentration. Dilution is when you add a solvent. Dilutions Definition Chemistry.