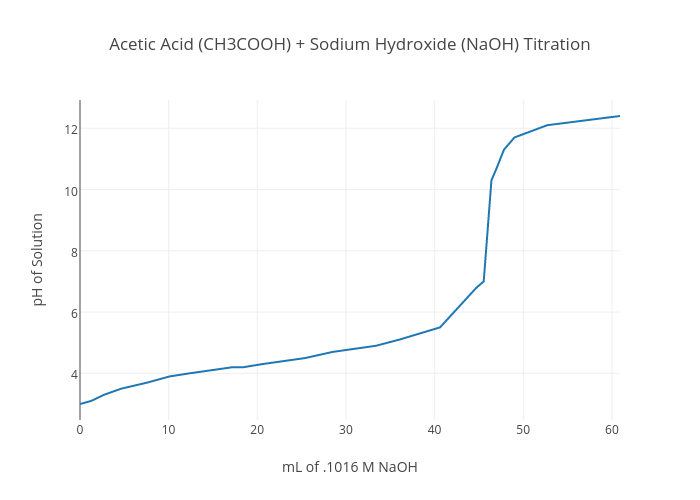
Suitable Indicator For Titration Between Ch3Cooh And Naoh . The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. If you look at the titration curve, which plots the volume of base added vs ph (source): The correct option is c. Examples of choosing an appropriate indicator for titrations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. You can see that the equivalence point occurs at ph = 7. This page assumes that you know about ph. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). Titration of ch 3 cooh against.
from mungfali.com
If you look at the titration curve, which plots the volume of base added vs ph (source): Titration of ch 3 cooh against. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. This page assumes that you know about ph. You can see that the equivalence point occurs at ph = 7. The correct option is c. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Examples of choosing an appropriate indicator for titrations. Both indicators change colour over a specific ph range.
Titration Curve Of Acetic Acid
Suitable Indicator For Titration Between Ch3Cooh And Naoh The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Examples of choosing an appropriate indicator for titrations. This page assumes that you know about ph. If you look at the titration curve, which plots the volume of base added vs ph (source): You can see that the equivalence point occurs at ph = 7. Both indicators change colour over a specific ph range. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). Titration of ch 3 cooh against. The correct option is c.
From pubs.sciepub.com
Figure 9. First derivative plot of the titration of weak acid (CH3COOH Suitable Indicator For Titration Between Ch3Cooh And Naoh Titration of ch 3 cooh against. Examples of choosing an appropriate indicator for titrations. You can see that the equivalence point occurs at ph = 7. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph. The graph shows the results obtained using two indicators (methyl red. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Suitable Indicator For Titration Between Ch3Cooh And Naoh This page assumes that you know about ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. If you look at the titration curve, which plots the volume of base added vs ph (source): Both. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.numerade.com
SOLVED Calculate the pH at the equivalence point for the titration of Suitable Indicator For Titration Between Ch3Cooh And Naoh Examples of choosing an appropriate indicator for titrations. Both indicators change colour over a specific ph range. Titration of ch 3 cooh against. The correct option is c. You can see that the equivalence point occurs at ph = 7. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.youtube.com
DAT Titration Curve of Weak Acid Strong Base (CH3COOH and NaOH Suitable Indicator For Titration Between Ch3Cooh And Naoh Titration of ch 3 cooh against. If you look at the titration curve, which plots the volume of base added vs ph (source): From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). You can see that the equivalence point occurs at ph =. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog Suitable Indicator For Titration Between Ch3Cooh And Naoh Titration of ch 3 cooh against. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From mungfali.com
Titration Curve Of Acetic Acid Suitable Indicator For Titration Between Ch3Cooh And Naoh From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). If you look at the titration curve, which plots the volume of base added vs ph (source): Examples of choosing an appropriate indicator for titrations. The two most common indicators that are used in. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Suitable Indicator For Titration Between Ch3Cooh And Naoh This page assumes that you know about ph. Examples of choosing an appropriate indicator for titrations. You can see that the equivalence point occurs at ph = 7. If you look at the titration curve, which plots the volume of base added vs ph (source): Both indicators change colour over a specific ph range. Titration of ch 3 cooh against.. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Suitable Indicator For Titration Between Ch3Cooh And Naoh Examples of choosing an appropriate indicator for titrations. The correct option is c. If you look at the titration curve, which plots the volume of base added vs ph (source): You can see that the equivalence point occurs at ph = 7. Both indicators change colour over a specific ph range. Titration of ch 3 cooh against. The two most. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Suitable Indicator For Titration Between Ch3Cooh And Naoh If you look at the titration curve, which plots the volume of base added vs ph (source): Titration of ch 3 cooh against. This page assumes that you know about ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Suitable Indicator For Titration Between Ch3Cooh And Naoh If you look at the titration curve, which plots the volume of base added vs ph (source): Titration of ch 3 cooh against. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). The two most common indicators that are used in titrations are. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free Suitable Indicator For Titration Between Ch3Cooh And Naoh Examples of choosing an appropriate indicator for titrations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Titration of ch 3 cooh against. You can see that the equivalence point occurs at ph = 7. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + CH3COOH = CH3COONa + H2O Suitable Indicator For Titration Between Ch3Cooh And Naoh The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. Both indicators change colour over a specific ph range. The correct option is c. Titration of ch 3 cooh against. Examples of choosing an appropriate indicator. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free Suitable Indicator For Titration Between Ch3Cooh And Naoh This page assumes that you know about ph. The correct option is c. Titration of ch 3 cooh against. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq).. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From klaimjxhd.blob.core.windows.net
Titration Between Hcl And Naoh at Tamika Spear blog Suitable Indicator For Titration Between Ch3Cooh And Naoh The correct option is c. Titration of ch 3 cooh against. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From loexhvzxl.blob.core.windows.net
Indicator For Ethanoic Acid And Sodium Hydroxide at Christopher Dent blog Suitable Indicator For Titration Between Ch3Cooh And Naoh This page assumes that you know about ph. The correct option is c. You can see that the equivalence point occurs at ph = 7. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. If you look at the titration curve, which plots the volume of base added vs ph (source): Titration of ch. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Suitable Indicator For Titration Between Ch3Cooh And Naoh You can see that the equivalence point occurs at ph = 7. Both indicators change colour over a specific ph range. If you look at the titration curve, which plots the volume of base added vs ph (source): The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.youtube.com
Acetic acid (ethanoic acid) and Sodium hydroxide reaction CH3COOH Suitable Indicator For Titration Between Ch3Cooh And Naoh Examples of choosing an appropriate indicator for titrations. This page assumes that you know about ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. You can see that the equivalence point occurs at ph. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Suitable Indicator For Titration Between Ch3Cooh And Naoh Examples of choosing an appropriate indicator for titrations. The correct option is c. If you look at the titration curve, which plots the volume of base added vs ph (source): The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.youtube.com
CH3COOH+NaOH=CH3COONa+H2O. balance the chemical equation Suitable Indicator For Titration Between Ch3Cooh And Naoh Both indicators change colour over a specific ph range. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). This page assumes that you know about ph. You can see that the equivalence point occurs at ph = 7. Titration of ch 3 cooh. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.numerade.com
SOLVED The following data is from a conductometric titration of 10.00 Suitable Indicator For Titration Between Ch3Cooh And Naoh Titration of ch 3 cooh against. If you look at the titration curve, which plots the volume of base added vs ph (source): Both indicators change colour over a specific ph range. This page assumes that you know about ph. The correct option is c. Examples of choosing an appropriate indicator for titrations. The graph shows the results obtained using. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From slideplayer.com
Neutralization of Acids and Bases ppt download Suitable Indicator For Titration Between Ch3Cooh And Naoh You can see that the equivalence point occurs at ph = 7. Examples of choosing an appropriate indicator for titrations. The correct option is c. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). Both indicators change colour over a specific ph range.. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.numerade.com
SOLVED A 0.100 M solution of ethanoic acid, CH3COOH, has a pH of 2.87 Suitable Indicator For Titration Between Ch3Cooh And Naoh Both indicators change colour over a specific ph range. You can see that the equivalence point occurs at ph = 7. Titration of ch 3 cooh against. Examples of choosing an appropriate indicator for titrations. If you look at the titration curve, which plots the volume of base added vs ph (source): From the results of parts c and d,. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Suitable Indicator For Titration Between Ch3Cooh And Naoh The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. This page. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Suitable Indicator For Titration Between Ch3Cooh And Naoh The correct option is c. If you look at the titration curve, which plots the volume of base added vs ph (source): Titration of ch 3 cooh against. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein,. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From cewxywne.blob.core.windows.net
AcidBase Titration Variables at Christopher Snodgrass blog Suitable Indicator For Titration Between Ch3Cooh And Naoh Both indicators change colour over a specific ph range. Examples of choosing an appropriate indicator for titrations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From dxonbtcto.blob.core.windows.net
Chemical Indicator AcidBase Titration at Hilda Johnston blog Suitable Indicator For Titration Between Ch3Cooh And Naoh Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. You can. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.numerade.com
SOLVED The distinctive odor of vinegar is due to acetic acid, CH3COOH Suitable Indicator For Titration Between Ch3Cooh And Naoh The correct option is c. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. You can see that the equivalence point occurs at ph = 7. This page assumes that you know about ph. Examples of choosing an appropriate indicator for titrations. From the results. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Suitable Indicator For Titration Between Ch3Cooh And Naoh The correct option is c. This page assumes that you know about ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. You can see that the equivalence point occurs at ph = 7. Titration. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From www.toppr.com
Match the titrations given with the indicators used in them Titration Suitable Indicator For Titration Between Ch3Cooh And Naoh The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The correct option is c. Examples of choosing an appropriate indicator for titrations. From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). If you look at the titration. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Suitable Indicator For Titration Between Ch3Cooh And Naoh The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. Both indicators change colour over a specific ph range. You can. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From selfdirectedce.com
How to Balance NaOH + CH3COOH = CH3COONa + H2O สังเคราะห์ข้อมูลที่ Suitable Indicator For Titration Between Ch3Cooh And Naoh Examples of choosing an appropriate indicator for titrations. If you look at the titration curve, which plots the volume of base added vs ph (source): The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The correct option is c. From the results of parts c and d, decide and explain which indicator, methyl orange. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From exoafzput.blob.core.windows.net
Titration Graph Point at Arleen McKoy blog Suitable Indicator For Titration Between Ch3Cooh And Naoh The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. The correct option is c. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid). Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From courses.lumenlearning.com
15.2 AcidBase Titrations Chemistry Suitable Indicator For Titration Between Ch3Cooh And Naoh The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. Both indicators change colour over a specific ph range. You can see that the equivalence point occurs at ph = 7. From the results of parts. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Suitable Indicator For Titration Between Ch3Cooh And Naoh The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. Both indicators change colour over a specific ph range. Titration of ch 3 cooh against. If you look at the titration curve, which plots the volume. Suitable Indicator For Titration Between Ch3Cooh And Naoh.
From ceplknbb.blob.core.windows.net
Acid Base Titration Questions at Deborah Wedel blog Suitable Indicator For Titration Between Ch3Cooh And Naoh From the results of parts c and d, decide and explain which indicator, methyl orange or phenolphthalein, is suitable for the titration between ch 3 cooh(aq). You can see that the equivalence point occurs at ph = 7. Titration of ch 3 cooh against. Both indicators change colour over a specific ph range. If you look at the titration curve,. Suitable Indicator For Titration Between Ch3Cooh And Naoh.