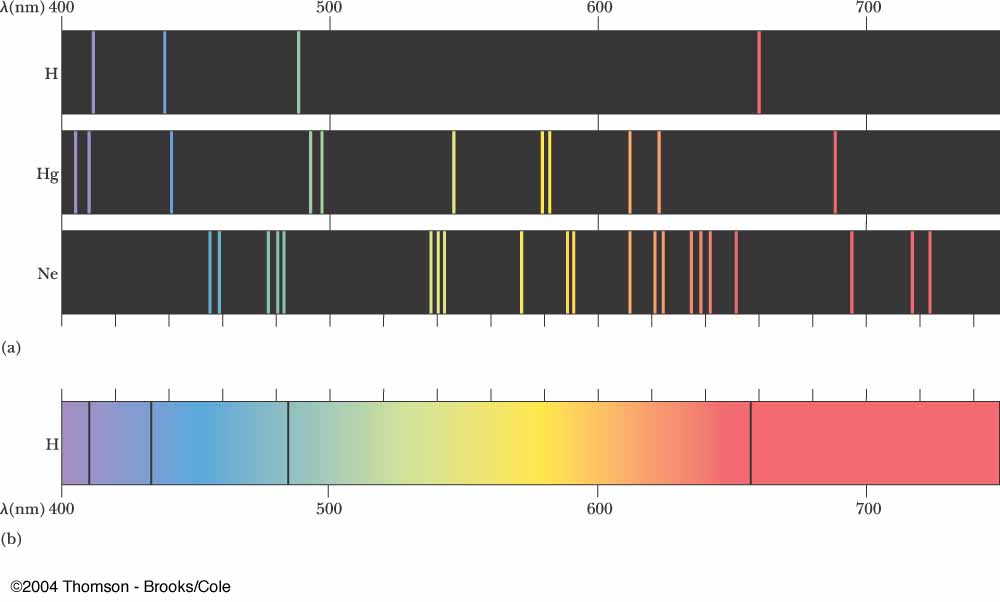
Emission Spectra Types . The shapes of these emission spectra fall into two broad types: Continuous spectra and discontinuous (or line) spectra. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. A continuous spectrum shows a. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. The characteristic spectrum of each element can be used in. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Atomic spectra can be broadly classified into two types based on their appearance:
from www.physics.umd.edu
A continuous spectrum shows a. Atomic spectra can be broadly classified into two types based on their appearance: Continuous spectra and discontinuous (or line) spectra. The shapes of these emission spectra fall into two broad types: An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The characteristic spectrum of each element can be used in. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. The result is an emission spectrum that shows the intensity of emission as a function of wavelength.
QUANTUM PHYSICS I
Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atomic spectra can be broadly classified into two types based on their appearance: An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The shapes of these emission spectra fall into two broad types: Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Continuous spectra and discontinuous (or line) spectra. A continuous spectrum shows a. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The characteristic spectrum of each element can be used in.
From www.slideserve.com
PPT EMISSION AND ABSORPTION SPECTRA PowerPoint Presentation, free Emission Spectra Types The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. A continuous spectrum shows a. The shapes of these emission spectra fall into two broad types: Atomic spectra can be broadly classified. Emission Spectra Types.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Types The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. Atomic spectra can be broadly classified into two types based on their appearance: An emission spectrum. Emission Spectra Types.
From jila.colorado.edu
4. Emission Spectra Types Atomic spectra can be broadly classified into two types based on their appearance: The characteristic spectrum of each element can be used in. Continuous spectra and discontinuous (or line) spectra. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. An emission spectrum. Emission Spectra Types.
From www.researchgate.net
Emission spectra of LED light sources. Download Scientific Diagram Emission Spectra Types Atomic spectra can be broadly classified into two types based on their appearance: An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Continuous spectra and discontinuous (or line) spectra. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. A. Emission Spectra Types.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Types Continuous spectra and discontinuous (or line) spectra. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Atomic spectra can be broadly classified into two types based on their appearance: The shapes. Emission Spectra Types.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. A continuous spectrum shows a. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The characteristic spectrum of each element can be used in. This spectrum of radiation emitted by. Emission Spectra Types.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Types A continuous spectrum shows a. Atomic spectra can be broadly classified into two types based on their appearance: Continuous spectra and discontinuous (or line) spectra. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. This spectrum of radiation emitted by electrons in. Emission Spectra Types.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atomic spectra can be broadly classified into two types based on their appearance: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. Emission Spectra Types.
From www.slideserve.com
PPT Three types of Spectra PowerPoint Presentation, free download Emission Spectra Types The characteristic spectrum of each element can be used in. A continuous spectrum shows a. The shapes of these emission spectra fall into two broad types: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. An emission spectrum of an element is. Emission Spectra Types.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Types Atomic spectra can be broadly classified into two types based on their appearance: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. This spectrum of. Emission Spectra Types.
From vqg1811-700.com
What are Absorption, Excitation and Emission Spectra? Tech Blog Emission Spectra Types The characteristic spectrum of each element can be used in. Atomic spectra can be broadly classified into two types based on their appearance: A continuous spectrum shows a. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. This spectrum of radiation emitted by electrons in the excited atoms. Emission Spectra Types.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Emission Spectra Types A continuous spectrum shows a. Continuous spectra and discontinuous (or line) spectra. The shapes of these emission spectra fall into two broad types: Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as. Emission Spectra Types.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Types A continuous spectrum shows a. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The characteristic spectrum of each element can be used in. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected. Emission Spectra Types.
From laderarctic.weebly.com
Emission spectra laderarctic Emission Spectra Types Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. An emission spectrum of an element is the unique pattern of light obtained when. Emission Spectra Types.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectra Types This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The characteristic spectrum of each element can be used in. The shapes of these. Emission Spectra Types.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and. Emission Spectra Types.
From www.researchgate.net
Typical emission spectra of three types of antiStokes luminescent Emission Spectra Types This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. The shapes of these emission spectra fall into two broad types: Atomic spectra can be broadly classified into two types. Emission Spectra Types.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Types This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Continuous spectra and discontinuous (or line) spectra. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. A continuous spectrum shows a. Emission and absorption spectra form the basis. Emission Spectra Types.
From www.researchgate.net
Light emission spectra in heavy water for probes 7 and 8 complexed with Emission Spectra Types Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The characteristic spectrum of each element can be used in. The. Emission Spectra Types.
From materialcampussteffen.z19.web.core.windows.net
Emission Spectra Worksheet Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The characteristic spectrum of each element can be used in. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. Continuous spectra and discontinuous (or line) spectra.. Emission Spectra Types.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra Types A continuous spectrum shows a. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and. Emission Spectra Types.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The shapes of these emission spectra fall into two broad types: A continuous spectrum shows a. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Atomic spectra can be broadly. Emission Spectra Types.
From www.howtuo.com
The Bright Lines Of An Emission Spectrum Are The Result Of • HOWTUO Emission Spectra Types The shapes of these emission spectra fall into two broad types: This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The result is. Emission Spectra Types.
From www.slideserve.com
PPT Basics of Astronomy PowerPoint Presentation, free download ID Emission Spectra Types The shapes of these emission spectra fall into two broad types: An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. A continuous spectrum shows a. Continuous spectra and discontinuous (or line) spectra. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star. Emission Spectra Types.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Emission Spectra Types A continuous spectrum shows a. The characteristic spectrum of each element can be used in. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The shapes of these. Emission Spectra Types.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Emission Spectra Types The characteristic spectrum of each element can be used in. The shapes of these emission spectra fall into two broad types: A continuous spectrum shows a. Atomic spectra can be broadly classified into two types based on their appearance: This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and. Emission Spectra Types.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. A continuous spectrum shows a. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about. Emission Spectra Types.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Emission Spectra Types Atomic spectra can be broadly classified into two types based on their appearance: An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Continuous spectra and discontinuous (or line) spectra. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. Emission Spectra Types.
From www.chegg.com
Solved A. Different Types of Spectra Identify each of the Emission Spectra Types This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. A continuous spectrum shows a. The characteristic spectrum of each element can be used in. Atomic spectra can be broadly classified into two types based on their appearance: The shapes of these emission spectra fall into two broad types: Continuous, emission,. Emission Spectra Types.
From www.chem1.com
Light, particles and waves Emission Spectra Types The characteristic spectrum of each element can be used in. Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. Continuous spectra and discontinuous (or line) spectra. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The shapes of. Emission Spectra Types.
From www.researchgate.net
a emission spectra; b normalized emission spectra; c UV spectrum (left Emission Spectra Types Continuous, emission, and absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas,. An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atomic spectra can be broadly classified into two types based on their appearance: The result is an. Emission Spectra Types.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectra Types The shapes of these emission spectra fall into two broad types: This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. Atomic spectra can. Emission Spectra Types.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Types Continuous spectra and discontinuous (or line) spectra. A continuous spectrum shows a. The shapes of these emission spectra fall into two broad types: The characteristic spectrum of each element can be used in. Atomic spectra can be broadly classified into two types based on their appearance: An emission spectrum of an element is the unique pattern of light obtained when. Emission Spectra Types.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Types The characteristic spectrum of each element can be used in. A continuous spectrum shows a. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The shapes of these emission spectra fall into two broad types: Continuous, emission, and absorption infographic showing the. Emission Spectra Types.
From www.physics.umd.edu
QUANTUM PHYSICS I Emission Spectra Types An emission spectrum of an element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atomic spectra can be broadly classified into two types based on their appearance: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. Emission Spectra Types.