Mg Element Ionization Energy . Km91a mg ii ground state 1s 2 2s 2 2p 6. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Ionization energy, also called ionization potential, is the energy necessary to remove an. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. Physical and chemical properties of magnesium: First ionization energy of magnesium is 7.6462 ev. 120 rows ionization energy chart of all the elements is given below. Molar ionization energies of the elements. This is the energy per.
from www.numerade.com
Km91a mg ii ground state 1s 2 2s 2 2p 6. First ionization energy, second ionization energy as well as. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. First ionization energy of magnesium is 7.6462 ev. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Ionization energy, also called ionization potential, is the energy necessary to remove an. Molar ionization energies of the elements. 120 rows ionization energy chart of all the elements is given below. These tables list values of molar ionization energies, measured in kj⋅mol −1. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state.
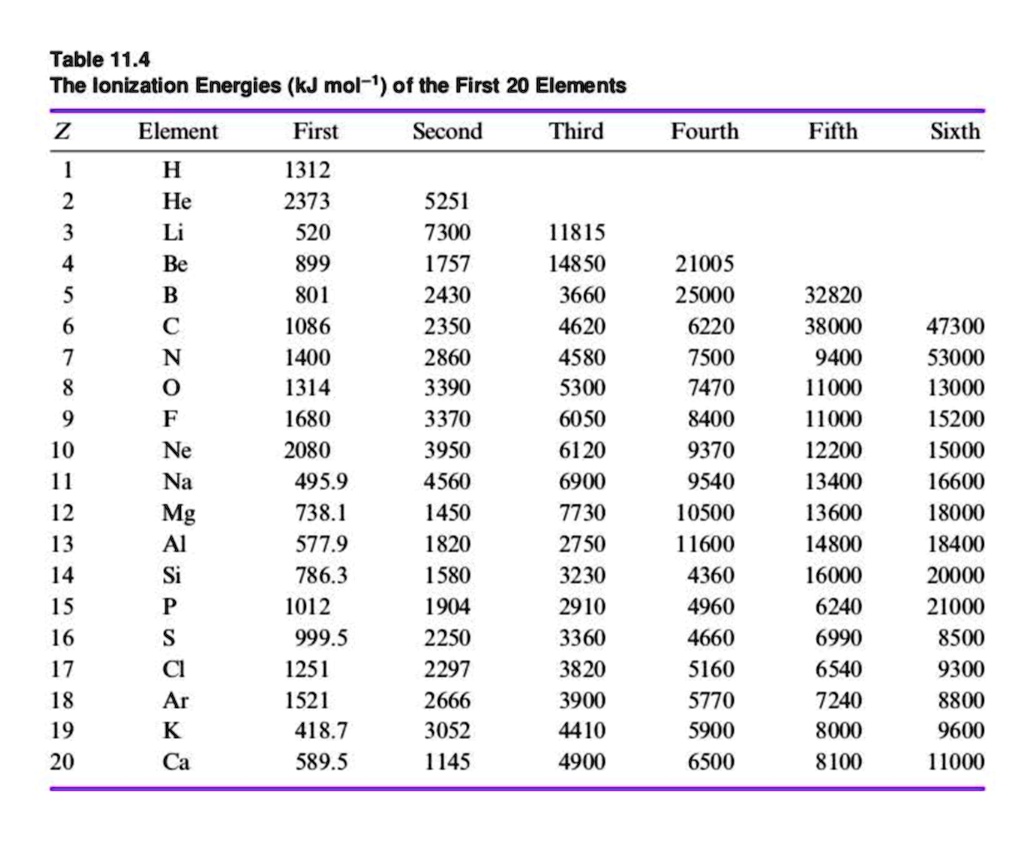
SOLVED Table 11.4 The Ionization Energies (kJ mol1) of the First 20
Mg Element Ionization Energy This is the energy per. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. First ionization energy of magnesium is 7.6462 ev. Ionization energy, also called ionization potential, is the energy necessary to remove an. General data, thermal properties, ionization energies, isotopes, reduction potentials,. These tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. This is the energy per. Molar ionization energies of the elements. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Km91a mg ii ground state 1s 2 2s 2 2p 6. Physical and chemical properties of magnesium:
From general.chemistrysteps.com
Ionization energy Chemistry Steps Mg Element Ionization Energy Physical and chemical properties of magnesium: In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Km91a mg ii ground state 1s 2 2s 2 2p 6. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Ionization energy, also called ionization potential, is the energy necessary to. Mg Element Ionization Energy.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Mg Element Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Km91a mg ii ground state 1s 2 2s 2 2p 6. These tables list values of molar ionization energies, measured in kj⋅mol −1. Chemists define the ionization energy (\(i\)) of an element as the amount of energy. Mg Element Ionization Energy.
From socratic.org
Which element has a higher 3rd ionization energy, Al or Mg? Why? Socratic Mg Element Ionization Energy Physical and chemical properties of magnesium: These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. Ionization energy, also called ionization potential, is the energy necessary to remove an. This is the energy per. First ionization energy of magnesium is 7.6462 ev. In physics and chemistry, ionization energy (ie). Mg Element Ionization Energy.
From www.chemistrylearner.com
Ionization Energy Definition, Chart & Periodic Table Trend Mg Element Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Physical and chemical properties of magnesium: Molar ionization energies of the elements. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Km91a mg ii ground state 1s 2 2s 2 2p 6.. Mg Element Ionization Energy.
From www.slideserve.com
PPT Aufbau Principle PowerPoint Presentation, free download ID5909290 Mg Element Ionization Energy Physical and chemical properties of magnesium: Molar ionization energies of the elements. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground. Mg Element Ionization Energy.
From www.chem1.com
Periodic properties of the elements Mg Element Ionization Energy Ionization energy, also called ionization potential, is the energy necessary to remove an. First ionization energy, second ionization energy as well as. Km91a mg ii ground state 1s 2 2s 2 2p 6. Physical and chemical properties of magnesium: General data, thermal properties, ionization energies, isotopes, reduction potentials,. Molar ionization energies of the elements. These tables list values of molar. Mg Element Ionization Energy.
From ar.inspiredpencil.com
Periodic Table Ionization Energy 3d Mg Element Ionization Energy These tables list values of molar ionization energies, measured in kj⋅mol −1. Physical and chemical properties of magnesium: General data, thermal properties, ionization energies, isotopes, reduction potentials,. Molar ionization energies of the elements. First ionization energy, second ionization energy as well as. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound. Mg Element Ionization Energy.
From www.slideserve.com
PPT Atomic Electron Configurations and Chemical Periodicity Mg Element Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. These tables list values of molar ionization energies, measured in kj⋅mol −1. Molar ionization energies of the elements. Km91a mg ii ground state 1s 2 2s 2 2p 6. In physics and. Mg Element Ionization Energy.
From www.sliderbase.com
Periodic Trends Presentation Chemistry Mg Element Ionization Energy First ionization energy of magnesium is 7.6462 ev. Physical and chemical properties of magnesium: This is the energy per. Molar ionization energies of the elements. These tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Km91a mg. Mg Element Ionization Energy.
From learningmagictorres.z21.web.core.windows.net
How To Identify Ionization Energy Mg Element Ionization Energy Physical and chemical properties of magnesium: First ionization energy of magnesium is 7.6462 ev. These tables list values of molar ionization energies, measured in kj⋅mol −1. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. First ionization energy, second ionization energy as well as. Km91a mg. Mg Element Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Mg Element Ionization Energy Km91a mg ii ground state 1s 2 2s 2 2p 6. These tables list values of molar ionization energies, measured in kj⋅mol −1. This is the energy per. Physical and chemical properties of magnesium: 120 rows ionization energy chart of all the elements is given below. Molar ionization energies of the elements. Ionization energy, also called ionization potential, is the. Mg Element Ionization Energy.
From www.toppr.com
First and second ionisation energies of magnesium are 7.646 and 15 Mg Element Ionization Energy Km91a mg ii ground state 1s 2 2s 2 2p 6. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. 120 rows ionization energy chart of all the elements is given below. Physical and chemical properties of magnesium: General data, thermal properties, ionization energies, isotopes, reduction potentials,. Chemists. Mg Element Ionization Energy.
From chemistnotes.com
Ionization energy and ionization potential Chemistry Notes Mg Element Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. These tables list values of molar ionization energies, measured in kj⋅mol −1. This is the energy per. Km91a mg ii ground state 1s 2 2s 2 2p 6. First ionization energy, second. Mg Element Ionization Energy.
From www.slideserve.com
PPT IONISATION ENERGY PowerPoint Presentation, free download ID3500637 Mg Element Ionization Energy General data, thermal properties, ionization energies, isotopes, reduction potentials,. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Km91a mg ii ground state 1s 2 2s 2 2p 6. This is the energy per. Physical and chemical properties of magnesium: 120 rows ionization energy chart of. Mg Element Ionization Energy.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID753034 Mg Element Ionization Energy First ionization energy, second ionization energy as well as. Physical and chemical properties of magnesium: These tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. Km91a mg ii ground state 1s 2 2s 2 2p 6. General data, thermal properties, ionization energies, isotopes, reduction potentials,. In. Mg Element Ionization Energy.
From www.slideserve.com
PPT Periodicity II PowerPoint Presentation, free download ID6839222 Mg Element Ionization Energy Molar ionization energies of the elements. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Ionization energy, also called ionization potential, is the energy necessary to remove an. Km91a mg ii ground state 1s 2 2s 2 2p 6. First ionization energy of magnesium is 7.6462 ev. Physical and chemical properties of magnesium: First ionization energy, second ionization energy as. Mg Element Ionization Energy.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Mg Element Ionization Energy Physical and chemical properties of magnesium: General data, thermal properties, ionization energies, isotopes, reduction potentials,. 120 rows ionization energy chart of all the elements is given below. Molar ionization energies of the elements. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. These tables list values. Mg Element Ionization Energy.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Mg Element Ionization Energy Molar ionization energies of the elements. 120 rows ionization energy chart of all the elements is given below. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Physical and chemical properties of magnesium: This is the energy per. Km91a mg ii. Mg Element Ionization Energy.
From awesomehome.co
Ionization Energy Table Of Elements Awesome Home Mg Element Ionization Energy 120 rows ionization energy chart of all the elements is given below. Molar ionization energies of the elements. This is the energy per. First ionization energy, second ionization energy as well as. Physical and chemical properties of magnesium: These tables list values of molar ionization energies, measured in kj⋅mol −1. In physics and chemistry, ionization energy (ie) is the minimum. Mg Element Ionization Energy.
From www.slideserve.com
PPT Ionization Energies and Group Numbers PowerPoint Presentation Mg Element Ionization Energy First ionization energy of magnesium is 7.6462 ev. 120 rows ionization energy chart of all the elements is given below. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Km91a mg ii ground state 1s 2 2s 2 2p 6. Ionization. Mg Element Ionization Energy.
From www.numerade.com
SOLVED Table 11.4 The Ionization Energies (kJ mol1) of the First 20 Mg Element Ionization Energy Ionization energy, also called ionization potential, is the energy necessary to remove an. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy of magnesium is 7.6462 ev. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. First ionization energy, second. Mg Element Ionization Energy.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Mg Element Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Ionization energy, also called ionization potential, is the energy necessary to remove an. This is the energy per. Molar ionization energies of the elements. In physics and chemistry, ionization energy (ie) is. Mg Element Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Mg Element Ionization Energy First ionization energy, second ionization energy as well as. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state.. Mg Element Ionization Energy.
From www.researchgate.net
Ionization energies of cations from groups Na⁺³, Mg⁺³, and Al⁺³ in Mg Element Ionization Energy First ionization energy of magnesium is 7.6462 ev. Molar ionization energies of the elements. This is the energy per. 120 rows ionization energy chart of all the elements is given below. Ionization energy, also called ionization potential, is the energy necessary to remove an. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most. Mg Element Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Mg Element Ionization Energy Ionization energy, also called ionization potential, is the energy necessary to remove an. Physical and chemical properties of magnesium: In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy. Mg Element Ionization Energy.
From www.periodic-table.org
Magnesium Ionization Energy Mg Element Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Molar ionization energies of the elements. These tables list values of molar ionization energies, measured in kj⋅mol −1. Km91a mg ii ground state 1s 2 2s 2 2p 6. First ionization energy of magnesium is 7.6462 ev.. Mg Element Ionization Energy.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Mg Element Ionization Energy General data, thermal properties, ionization energies, isotopes, reduction potentials,. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state.. Mg Element Ionization Energy.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Mg Element Ionization Energy 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Molar ionization energies of the elements. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Ionization energy, also called ionization potential, is the energy necessary to remove an. Km91a mg ii ground state 1s 2 2s 2 2p 6.. Mg Element Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Mg Element Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. 120 rows ionization energy chart of all the elements is given below. First ionization energy of magnesium is 7.6462 ev. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove. Mg Element Ionization Energy.
From www.slideserve.com
PPT Electron Configurations Chemical Periodicity (Ch 8) PowerPoint Mg Element Ionization Energy First ionization energy, second ionization energy as well as. Molar ionization energies of the elements. First ionization energy of magnesium is 7.6462 ev. Physical and chemical properties of magnesium: These tables list values of molar ionization energies, measured in kj⋅mol −1. Ionization energy, also called ionization potential, is the energy necessary to remove an. Km91a mg ii ground state 1s. Mg Element Ionization Energy.
From www.vrogue.co
Periodic Table Of Elements Energy Levels Periodic Tab vrogue.co Mg Element Ionization Energy Km91a mg ii ground state 1s 2 2s 2 2p 6. First ionization energy of magnesium is 7.6462 ev. First ionization energy, second ionization energy as well as. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Physical and chemical properties of magnesium: Chemists define the. Mg Element Ionization Energy.
From www.slideserve.com
PPT Atomic Electron Configurations and Chemical Periodicity Mg Element Ionization Energy First ionization energy of magnesium is 7.6462 ev. This is the energy per. First ionization energy, second ionization energy as well as. Physical and chemical properties of magnesium: In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Km91a mg ii ground state 1s 2 2s 2. Mg Element Ionization Energy.
From pubchem.ncbi.nlm.nih.gov
Ionization Energy Periodic Table of Elements PubChem Mg Element Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. This is the energy per. Molar ionization energies of the elements. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an. Mg Element Ionization Energy.
From www.youtube.com
Ionization Energy Basic Introduction YouTube Mg Element Ionization Energy Physical and chemical properties of magnesium: First ionization energy, second ionization energy as well as. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. First ionization energy of magnesium is 7.6462 ev.. Mg Element Ionization Energy.
From commons.wikimedia.org
Original file (SVG file, nominally 851 × 520 pixels, file size 91 KB) Mg Element Ionization Energy 120 rows ionization energy chart of all the elements is given below. First ionization energy of magnesium is 7.6462 ev. General data, thermal properties, ionization energies, isotopes, reduction potentials,. Physical and chemical properties of magnesium: This is the energy per. First ionization energy, second ionization energy as well as. Molar ionization energies of the elements. Km91a mg ii ground state. Mg Element Ionization Energy.